Multiple membrane interactions and versatile vesicle deformations elicited by melittin
- PMID: 23594437
- PMCID: PMC3705284
- DOI: 10.3390/toxins5040637
Multiple membrane interactions and versatile vesicle deformations elicited by melittin
Abstract
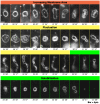
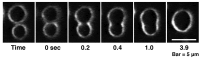
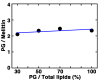
Melittin induces various reactions in membranes and has been widely studied as a model for membrane-interacting peptide; however, the mechanism whereby melittin elicits its effects remains unclear. Here, we observed melittin-induced changes in individual giant liposomes using direct real-time imaging by dark-field optical microscopy, and the mechanisms involved were correlated with results obtained using circular dichroism, cosedimentation, fluorescence quenching of tryptophan residues, and electron microscopy. Depending on the concentration of negatively charged phospholipids in the membrane and the molecular ratio between lipid and melittin, melittin induced the "increasing membrane area", "phased shrinkage", or "solubilization" of liposomes. In phased shrinkage, liposomes formed small particles on their surface and rapidly decreased in size. Under conditions in which the increasing membrane area, phased shrinkage, or solubilization were mainly observed, the secondary structure of melittin was primarily estimated as an α-helix, β-like, or disordered structure, respectively. When the increasing membrane area or phased shrinkage occurred, almost all melittin was bound to the membranes and reached more hydrophobic regions of the membranes than when solubilization occurred. These results indicate that the various effects of melittin result from its ability to adopt various structures and membrane-binding states depending on the conditions.
Figures











References
-
- Habermann E. Bee and wasp venoms. Science. 1972;177:314–321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

