Knockdown of the candidate dyslexia susceptibility gene homolog dyx1c1 in rodents: effects on auditory processing, visual attention, and cortical and thalamic anatomy
- PMID: 23594585
- PMCID: PMC3980864
- DOI: 10.1159/000348431
Knockdown of the candidate dyslexia susceptibility gene homolog dyx1c1 in rodents: effects on auditory processing, visual attention, and cortical and thalamic anatomy
Abstract
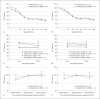
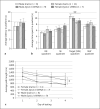
The current study investigated the behavioral and neuroanatomical effects of embryonic knockdown of the candidate dyslexia susceptibility gene (CDSG) homolog Dyx1c1 through RNA interference (RNAi) in rats. Specifically, we examined long-term effects on visual attention abilities in male rats, in addition to assessing rapid and complex auditory processing abilities in male and, for the first time, female rats. Our results replicated prior evidence of complex acoustic processing deficits in Dyx1c1 male rats and revealed new evidence of comparable deficits in Dyx1c1 female rats. Moreover, we found new evidence that knocking down Dyx1c1 produced orthogonal impairments in visual attention in the male subgroup. Stereological analyses of male brains from prior RNAi studies revealed that, despite consistent visible evidence of disruptions of neuronal migration (i.e., heterotopia), knockdown of Dyx1c1 did not significantly alter the cortical volume, hippocampal volume, or midsagittal area of the corpus callosum (measured in a separate cohort of like-treated Dyx1c1 male rats). Dyx1c1 transfection did, however, lead to significant changes in medial geniculate nucleus (MGN) anatomy, with a significant shift to smaller MGN neurons in Dyx1c1-transfected animals. Combined results provide important information about the impact of Dyx1c1 on behavioral functions that parallel domains known to be affected in language-impaired populations as well as information about widespread changes to the brain following early disruption of this CDSG.
Copyright © 2013 S. Karger AG, Basel.
Figures




Similar articles
-
Developmental disruptions and behavioral impairments in rats following in utero RNAi of Dyx1c1.Brain Res Bull. 2007 Mar 15;71(5):508-14. doi: 10.1016/j.brainresbull.2006.11.005. Epub 2006 Dec 5. Brain Res Bull. 2007. PMID: 17259020 Free PMC article.
-
Persistent spatial working memory deficits in rats following in utero RNAi of Dyx1c1.Genes Brain Behav. 2011 Mar;10(2):244-52. doi: 10.1111/j.1601-183X.2010.00662.x. Epub 2010 Nov 25. Genes Brain Behav. 2011. PMID: 20977651 Free PMC article.
-
The effects of embryonic knockdown of the candidate dyslexia susceptibility gene homologue Dyx1c1 on the distribution of GABAergic neurons in the cerebral cortex.Neuroscience. 2011 Jan 13;172:535-46. doi: 10.1016/j.neuroscience.2010.11.002. Epub 2010 Nov 9. Neuroscience. 2011. PMID: 21070838 Free PMC article.
-
Neocortical disruption and behavioral impairments in rats following in utero RNAi of candidate dyslexia risk gene Kiaa0319.Int J Dev Neurosci. 2012 Jun;30(4):293-302. doi: 10.1016/j.ijdevneu.2012.01.009. Epub 2012 Feb 3. Int J Dev Neurosci. 2012. PMID: 22326444 Free PMC article.
-
Early neural disruption and auditory processing outcomes in rodent models: implications for developmental language disability.Front Syst Neurosci. 2013 Oct 21;7:58. doi: 10.3389/fnsys.2013.00058. Front Syst Neurosci. 2013. PMID: 24155699 Free PMC article. Review.
Cited by
-
The dyslexia susceptibility KIAA0319 gene shows a specific expression pattern during zebrafish development supporting a role beyond neuronal migration.J Comp Neurol. 2019 Nov 1;527(16):2634-2643. doi: 10.1002/cne.24696. Epub 2019 Apr 16. J Comp Neurol. 2019. PMID: 30950042 Free PMC article.
-
Normal radial migration and lamination are maintained in dyslexia-susceptibility candidate gene homolog Kiaa0319 knockout mice.Brain Struct Funct. 2017 Apr;222(3):1367-1384. doi: 10.1007/s00429-016-1282-1. Epub 2016 Aug 10. Brain Struct Funct. 2017. PMID: 27510895 Free PMC article.
-
Neurobiological Sex Differences in Developmental Dyslexia.Front Psychol. 2019 Jan 11;9:2669. doi: 10.3389/fpsyg.2018.02669. eCollection 2018. Front Psychol. 2019. PMID: 30687153 Free PMC article.
-
An assessment of gene-by-gene interactions as a tool to unfold missing heritability in dyslexia.Hum Genet. 2015 Jul;134(7):749-60. doi: 10.1007/s00439-015-1555-4. Epub 2015 Apr 28. Hum Genet. 2015. PMID: 25916574 Clinical Trial.
-
Auditory processing enhancements in the TS2-neo mouse model of Timothy Syndrome, a rare genetic disorder associated with autism spectrum disorders.Adv Neurodev Disord. 2017 Sep;1(3):176-189. doi: 10.1007/s41252-017-0029-1. Epub 2017 Jul 1. Adv Neurodev Disord. 2017. PMID: 29159279 Free PMC article. No abstract available.
References
-
- Melby-Lervag M, Lyster SA, Hulme C. Phonological skills and their role in learning to read: a meta-analytic review. Psychol Bull. 2012;138:322–352. - PubMed
-
- Peyrin C, Lallier M, Demonet JF, Pernet C, Baciu M, Le Bas JF, Valdois S. Neural dissociation of phonological and visual attention span disorders in developmental dyslexia: FMRI evidence from two case reports. Brain Lang. 2012;120:381–94. - PubMed
-
- Beneventi H, Tonnessen FE, Ersland L, Hugdahl K. Executive working memory processes in dyslexia: Behavioral and fMRI evidence. Scand J Psychol. 2010;51:192–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

