Iron and cancer: more ore to be mined
- PMID: 23594855
- PMCID: PMC4036554
- DOI: 10.1038/nrc3495
Iron and cancer: more ore to be mined
Abstract
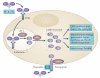
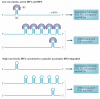
Iron is an essential nutrient that facilitates cell proliferation and growth. However, iron also has the capacity to engage in redox cycling and free radical formation. Therefore, iron can contribute to both tumour initiation and tumour growth; recent work has also shown that iron has a role in the tumour microenvironment and in metastasis. Pathways of iron acquisition, efflux, storage and regulation are all perturbed in cancer, suggesting that reprogramming of iron metabolism is a central aspect of tumour cell survival. Signalling through hypoxia-inducible factor (HIF) and WNT pathways may contribute to altered iron metabolism in cancer. Targeting iron metabolic pathways may provide new tools for cancer prognosis and therapy.
Figures

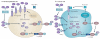


References
-
- Crichton R. Iron Metabolism: from Molecular Mechanisms to Cinical Consequences. John Wiley and Sons; 2009. pp. 17–58.
-
- Inoue S, Kawanishi S. Hydroxyl radical production and human DNA damage induced by ferric nitrilotriacetate and hydrogen peroxide. Cancer Res. 1987;47:6522–6527. - PubMed
-
- Dizdaroglu M, Rao G, Halliwell B, Gajewski E. Damage to the DNA bases in mammalian chromatin by hydrogen peroxide in the presence of ferric and cupric ions. Arch. Biochem. Biophys. 1991;285:317–324. - PubMed
-
- Dizdaroglu M, Jaruga P. Mechanisms of free radical-induced damage to DNA. Free Radic. Res. 2012;46:382–419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

