Computational solutions for omics data
- PMID: 23594911
- PMCID: PMC3966295
- DOI: 10.1038/nrg3433
Computational solutions for omics data
Abstract
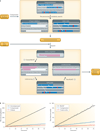
High-throughput experimental technologies are generating increasingly massive and complex genomic data sets. The sheer enormity and heterogeneity of these data threaten to make the arising problems computationally infeasible. Fortunately, powerful algorithmic techniques lead to software that can answer important biomedical questions in practice. In this Review, we sample the algorithmic landscape, focusing on state-of-the-art techniques, the understanding of which will aid the bench biologist in analysing omics data. We spotlight specific examples that have facilitated and enriched analyses of sequence, transcriptomic and network data sets.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

