Higher serum 25-hydroxyvitamin D concentrations associate with a faster recovery of skeletal muscle strength after muscular injury
- PMID: 23595134
- PMCID: PMC3705346
- DOI: 10.3390/nu5041253
Higher serum 25-hydroxyvitamin D concentrations associate with a faster recovery of skeletal muscle strength after muscular injury
Abstract
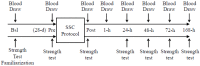
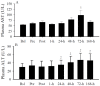
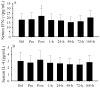
The primary purpose of this study was to identify if serum 25-hydroxyvitamin D (25(OH)D) concentrations predict muscular weakness after intense exercise. We hypothesized that pre-exercise serum 25(OH)D concentrations inversely predict exercise-induced muscular weakness. Fourteen recreationally active adults participated in this study. Each subject had one leg randomly assigned as a control. The other leg performed an intense exercise protocol. Single-leg peak isometric force and blood 25(OH)D, aspartate and alanine aminotransferases, albumin, interferon (IFN)-γ, and interleukin-4 were measured prior to and following intense exercise. Following exercise, serum 25(OH)D concentrations increased (p < 0.05) immediately, but within minutes, subsequently decreased (p < 0.05). Circulating albumin increases predicted (p < 0.005) serum 25(OH)D increases, while IFN-γ increases predicted (p < 0.001) serum 25(OH)D decreases. Muscular weakness persisted within the exercise leg (p < 0.05) and compared to the control leg (p < 0.05) after the exercise protocol. Serum 25(OH)D concentrations inversely predicted (p < 0.05) muscular weakness (i.e., control leg vs. exercise leg peak isometric force) immediately and days (i.e., 48-h and 72-h) after exercise, suggesting the attenuation of exercise-induced muscular weakness with increasing serum 25(OH)D prior to exercise. Based on these data, we conclude that pre-exercise serum 25(OH)D concentrations could influence the recovery of skeletal muscle strength after an acute bout of intense exercise.
Figures




References
-
- Jones G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 2008;88:582S–586S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

