Host plant-driven sensory specialization in Drosophila erecta
- PMID: 23595274
- PMCID: PMC3652467
- DOI: 10.1098/rspb.2013.0626
Host plant-driven sensory specialization in Drosophila erecta
Abstract
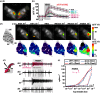
Finding appropriate feeding and breeding sites is crucial for all insects. To fulfil this vital task, many insects rely on their sense of smell. Alterations in the habitat--or in lifestyle--should accordingly also be reflected in the olfactory system. Solid functional evidence for direct adaptations in the olfactory system is however scarce. We have, therefore, examined the sense of smell of Drosophila erecta, a close relative of Drosophila melanogaster and specialist on screw pine fruits (Pandanus spp.). In comparison with three sympatric sibling species, D. erecta shows specific alterations in its olfactory system towards detection and processing of a characteristic Pandanus volatile (3-methyl-2-butenyl acetate, 3M2BA). We show that D. erecta is more sensitive towards this substance, and that the increased sensitivity derives from a numerical increase of one olfactory sensory neuron (OSN) class. We also show that axons from these OSNs form a complex of enlarged glomeruli in the antennal lobe, the first olfactory brain centre, of D. erecta. Finally, we show that 3M2BA induces oviposition in D. erecta, but not in D. melanogaster. The presumed adaptations observed here follow to a remarkable degree those found in Drosophila sechellia, a specialist upon noni fruit, and suggest a general principle for how specialization affects the sense of smell.
Figures


References
-
- Hansson BS, Stensmyr MC. 2011. Evolution of insect olfaction. Neuron 72, 698–71110.1016/j.neuron.2011.11.003 (doi:10.1016/j.neuron.2011.11.003) - DOI - DOI - PubMed
-
- McBride CS. 2007. Rapid evolution of smell and taste receptor genes during host specialization in Drosophila sechellia. Proc. Natl Acad. Sci. USA 104, 4996–500110.1073/pnas.0608424104 (doi:10.1073/pnas.0608424104) - DOI - DOI - PMC - PubMed
-
- Dekker T, Ibba I, Siju KP, Stensmyr MC, Hansson BS. 2006. Olfactory shifts parallel superspecialism for toxic fruit in Drosophila melanogaster sibling, D. sechellia. Curr. Biol. 16, 101–10910.1016/j.cub.2005.11.075 (doi:10.1016/j.cub.2005.11.075) - DOI - DOI - PubMed
-
- Stensmyr MC, Dekker T, Hansson BS. 2003. Evolution of the olfactory code in the Drosophila melanogaster subgroup. Proc. R. Soc. Lond. B 270, 2333–234010.1098/rspb.2003.2512 (doi:10.1098/rspb.2003.2512) - DOI - DOI - PMC - PubMed
-
- Ibba I, Angioy A, Hansson B, Dekker T. 2010. Macroglomeruli for fruit odors change blend preference in Drosophila. Naturwissenschaften 97, 1059–106610.1007/s00114-010-0727-2 (doi:10.1007/s00114-010-0727-2) - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

