Insufficient OPC migration into demyelinated lesions is a cause of poor remyelination in MS and mouse models
- PMID: 23595275
- PMCID: PMC3661931
- DOI: 10.1007/s00401-013-1112-y
Insufficient OPC migration into demyelinated lesions is a cause of poor remyelination in MS and mouse models
Abstract
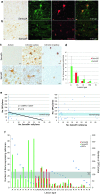
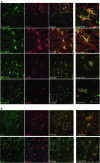
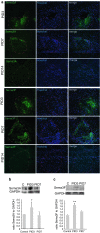
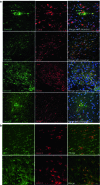
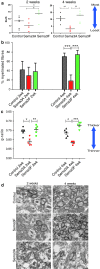
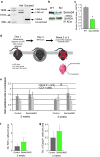
Failure of remyelination of multiple sclerosis (MS) lesions contributes to neurodegeneration that correlates with chronic disability in patients. Currently, there are no available treatments to reduce neurodegeneration, but one therapeutic approach to fill this unmet need is to promote remyelination. As many demyelinated MS lesions contain plentiful oligodendrocyte precursor cells (OPCs), but no mature myelinating oligodendrocytes, research has previously concentrated on promoting OPC maturation. However, some MS lesions contain few OPCs, and therefore, remyelination failure may also be secondary to OPC recruitment failure. Here, in a series of MS samples, we determined how many lesions contained few OPCs, and correlated this to pathological subtype and expression of the chemotactic molecules Semaphorin (Sema) 3A and 3F. 37 % of MS lesions contained low numbers of OPCs, and these were mostly chronic active lesions, in which cells expressed Sema3A (chemorepellent). To test the hypothesis that differential Sema3 expression in demyelinated lesions alters OPC recruitment and the efficiency of subsequent remyelination, we used a focal myelinotoxic mouse model of demyelination. Adding recombinant (r)Sema3A (chemorepellent) to demyelinated lesions reduced OPC recruitment and remyelination, whereas the addition of rSema3F (chemoattractant), or use of transgenic mice with reduced Sema3A expression increased OPC recruitment and remyelination. We conclude that some MS lesions fail to remyelinate secondary to reduced OPC recruitment, and that chemotactic molecules are involved in the mechanism, providing a new group of drug targets to improve remyelination, with a specific target in the Sema3A receptor neuropilin-1.
Trial registration: ClinicalTrials.gov NCT01244139.
Figures





References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

