Mild traumatic brain injury in the mouse induces axotomy primarily within the axon initial segment
- PMID: 23595276
- PMCID: PMC3691315
- DOI: 10.1007/s00401-013-1119-4
Mild traumatic brain injury in the mouse induces axotomy primarily within the axon initial segment
Abstract
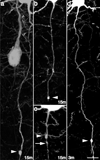
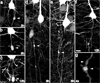
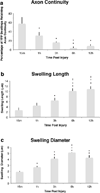
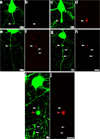
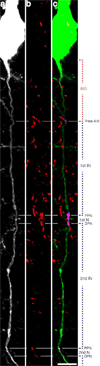
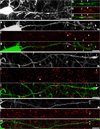
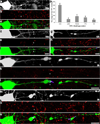
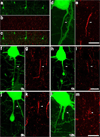
Traumatic axonal injury (TAI) is a consistent component of traumatic brain injury (TBI), and is associated with much of its morbidity. Increasingly, it has also been recognized as a major pathology of mild TBI (mTBI). In terms of its pathogenesis, numerous studies have investigated the susceptibility of the nodes of Ranvier, the paranode and internodal regions to TAI. The nodes of Ranvier, with their unique composition and concentration of ion channels, have been suggested as the primary site of injury, initiating a cascade of abnormalities in the related paranodal and internodal domains that lead to local axonal swellings and detachment. No investigation, however, has determined the effect of TAI upon the axon initial segment (AIS), a segment critical to regulating polarity and excitability. The current study sought to identify the susceptibility of these different axon domains to TAI within the neocortex, where each axonal domain could be simultaneously assessed. Utilizing a mouse model of mTBI, a temporal and spatial heterogeneity of axonal injury was found within the neocortical gray matter. Although axonal swellings were found in all domains along myelinated neocortical axons, the majority of TAI occurred within the AIS, which progressed without overt structural disruption of the AIS itself. The finding of primary AIS involvement has important implications regarding neuronal polarity and the fate of axotomized processes, while also raising therapeutic implications, as the mechanisms underlying such axonal injury in the AIS may be distinct from those described for nodal/paranodal injury.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15(1):49–59. - PubMed
-
- Banik NL, Matzelle DC, Gantt-Wilford G, Osborne A, Hogan EL. Increased calpain content and progressive degradation of neurofilament protein in spinal cord injury. Brain Res. 1997;752(1–2):301–306. - PubMed
-
- Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma. 2007;24(9):1447–1459. - PubMed
-
- Bender KJ, Trussell LO. The physiology of the axon initial segment. Annu Rev Neurosci. 2012;35:249–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

