Evaluation of RevA, a fibronectin-binding protein of Borrelia burgdorferi, as a potential vaccine candidate for lyme disease
- PMID: 23595502
- PMCID: PMC3675963
- DOI: 10.1128/CVI.00758-12
Evaluation of RevA, a fibronectin-binding protein of Borrelia burgdorferi, as a potential vaccine candidate for lyme disease
Abstract
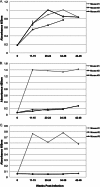
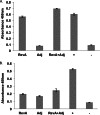
Previous studies indicated that the Lyme disease spirochete Borrelia burgdorferi expresses the RevA outer surface protein during mammalian infection. As an adhesin that promotes bacterial interaction with fibronectin, RevA appears to be a good target for preventive therapies. RevA proteins are highly conserved across all Lyme borreliae, and antibodies against RevA protein are cross-reactive among RevA proteins from diverse strains. Mice infected with B. burgdorferi mounted a rapid IgM response to RevA, followed by a strong IgG response that generally remained elevated for more than 12 months, suggesting continued exposure of RevA protein to the immune system. RevA antibodies were bactericidal in vitro. To evaluate the RevA antigen as a potential vaccine, mice were vaccinated with recombinant RevA and challenged with B. burgdorferi by inoculation with a needle or by a tick bite. Cultured tissues from all treatment groups were positive for B. burgdorferi. Vaccinated animals also appeared to have similar levels of B. burgdorferi DNA compared to nonvaccinated controls. Despite its antigenicity, surface expression, and the production of bactericidal antibodies against it, RevA does not protect against Borrelia burgdorferi infection in a mouse model. However, passive immunization with anti-RevA antibodies did prevent infection, suggesting the possible utility of RevA-based immunotherapeutics or vaccine.
Figures




Similar articles
-
Tick-Tattoo: DNA Vaccination Against B. burgdorferi or Ixodes scapularis Tick Proteins.Front Immunol. 2021 Feb 25;12:615011. doi: 10.3389/fimmu.2021.615011. eCollection 2021. Front Immunol. 2021. PMID: 33717102 Free PMC article.
-
Identification of Surface Epitopes Associated with Protection against Highly Immune-Evasive VlsE-Expressing Lyme Disease Spirochetes.Infect Immun. 2018 Jul 23;86(8):e00182-18. doi: 10.1128/IAI.00182-18. Print 2018 Aug. Infect Immun. 2018. PMID: 29866906 Free PMC article.
-
Long-term protection of mice from Lyme disease by vaccination with OspA.Infect Immun. 1992 Mar;60(3):773-7. doi: 10.1128/iai.60.3.773-777.1992. Infect Immun. 1992. PMID: 1541551 Free PMC article.
-
A recombinant vaccine for Lyme disease.Behring Inst Mitt. 1994 Dec;(95):106-8. Behring Inst Mitt. 1994. PMID: 7755503 Review.
-
Vaccination as a modality to prevent Lyme disease. A status report.Infect Dis Clin North Am. 1999 Mar;13(1):135-48, vii. doi: 10.1016/s0891-5520(05)70047-7. Infect Dis Clin North Am. 1999. PMID: 10198796 Review.
Cited by
-
Borrelia peptidoglycan interacting Protein (BpiP) contributes to the fitness of Borrelia burgdorferi against host-derived factors and influences virulence in mouse models of Lyme disease.PLoS Pathog. 2021 Apr 21;17(4):e1009535. doi: 10.1371/journal.ppat.1009535. eCollection 2021 Apr. PLoS Pathog. 2021. PMID: 33882111 Free PMC article.
-
That's my story, and I'm sticking to it--an update on B. burgdorferi adhesins.Front Cell Infect Microbiol. 2014 Apr 3;4:41. doi: 10.3389/fcimb.2014.00041. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24772392 Free PMC article. Review.
-
Structure-function analysis of PorXFj, the PorX homolog from Flavobacterium johnsioniae, suggests a role of the CheY-like domain in type IX secretion motor activity.Sci Rep. 2024 Mar 19;14(1):6577. doi: 10.1038/s41598-024-57089-9. Sci Rep. 2024. PMID: 38503809 Free PMC article.
-
Borrelia burgdorferi RevA Significantly Affects Pathogenicity and Host Response in the Mouse Model of Lyme Disease.Infect Immun. 2015 Sep;83(9):3675-83. doi: 10.1128/IAI.00530-15. Epub 2015 Jul 6. Infect Immun. 2015. PMID: 26150536 Free PMC article.
-
Borrelia surface proteins: new horizons in Lyme disease diagnosis.Appl Microbiol Biotechnol. 2025 Jul 1;109(1):156. doi: 10.1007/s00253-025-13490-6. Appl Microbiol Biotechnol. 2025. PMID: 40590992 Free PMC article. Review.
References
-
- Centers for Disease Control and Prevention 2011. Summary of notifiable diseases—United States, 2009. Morb. Mortal. Wkly. Rep. 58:1–100 - PubMed
-
- Stanek G, Wormser GP, Gray J, Strle F. 2012. Lyme borreliosis. Lancet 379:461–473 - PubMed
-
- Stanek G, Strle F. 2003. Lyme borreliosis. Lancet 362:1639–1647 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

