The iron metallome in eukaryotic organisms
- PMID: 23595675
- PMCID: PMC3924584
- DOI: 10.1007/978-94-007-5561-1_8
The iron metallome in eukaryotic organisms
Abstract
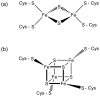
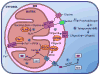
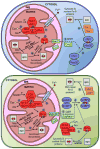
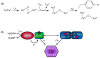
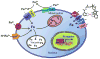
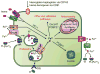
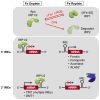
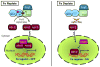
This chapter is focused on the iron metallome in eukaryotes at the cellular and subcellular level, including properties, utilization in metalloproteins, trafficking, storage, and regulation of these processes. Studies in the model eukaryote Saccharomyces cerevisiae and mammalian cells will be highlighted. The discussion of iron properties will center on the speciation and localization of intracellular iron as well as the cellular and molecular mechanisms for coping with both low iron bioavailability and iron toxicity. The section on iron metalloproteins will emphasize heme, iron-sulfur cluster, and non-heme iron centers, particularly their cellular roles and mechanisms of assembly. The section on iron uptake, trafficking, and storage will compare methods used by yeast and mammalian cells to import iron, how this iron is brought into various organelles, and types of iron storage proteins. Regulation of these processes will be compared between yeast and mammalian cells at the transcriptional, post-transcriptional, and post-translational levels.
Figures
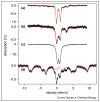
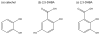
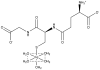
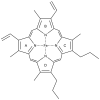
References
-
- Epsztejn S, Glickstein H, Picard V, Slotki IN, Breuer W, Beaumont C, Cabantchik ZI. Blood. 1999;94:3593–3603. - PubMed
-
- Petrat F, Rauen U, de Groot H. Hepatology. 1999;29:1171–1179. - PubMed
-
- Ceccarelli D, Gallesi D, Giovannini F, Ferrali M, Masini A. Biochem Biophys Res Commun. 1995;209:53–59. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

