Sodium channel cleavage is associated with aberrant neuronal activity and cognitive deficits in a mouse model of Alzheimer's disease
- PMID: 23595759
- PMCID: PMC6618875
- DOI: 10.1523/JNEUROSCI.2325-12.2013
Sodium channel cleavage is associated with aberrant neuronal activity and cognitive deficits in a mouse model of Alzheimer's disease
Erratum in
- J Neurosci. 2013 Jun 26;33(26):10934. Brown, Jon T [added]; Randall, Andrew D [added]
Abstract
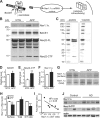
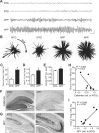
BACE1 is the rate-limiting enzyme that cleaves amyloid precursor protein (APP) to produce the amyloid β peptides that accumulate in Alzheimer's disease (AD). BACE1, which is elevated in AD patients and APP transgenic mice, also cleaves the β2-subunit of voltage-gated sodium channels (Navβ2). Although increased BACE1 levels are associated with Navβ2 cleavage in AD patients, whether Navβ2 cleavage occurs in APP mice had not yet been examined. Such a finding would be of interest because of its potential impact on neuronal activity: previous studies demonstrated that BACE1-overexpressing mice exhibit excessive cleavage of Navβ2 and reduced sodium current density, but the phenotype associated with loss of function mutations in either Navβ-subunits or pore-forming α-subunits is epilepsy. Because mounting evidence suggests that epileptiform activity may play an important role in the development of AD-related cognitive deficits, we examined whether enhanced cleavage of Navβ2 occurs in APP transgenic mice, and whether it is associated with aberrant neuronal activity and cognitive deficits. We found increased levels of BACE1 expression and Navβ2 cleavage fragments in cortical lysates from APP transgenic mice, as well as associated alterations in Nav1.1α expression and localization. Both pyramidal neurons and inhibitory interneurons exhibited evidence of increased Navβ2 cleavage. Moreover, the magnitude of alterations in sodium channel subunits was associated with aberrant EEG activity and impairments in the Morris water maze. Together, these results suggest that altered processing of voltage-gated sodium channels may contribute to aberrant neuronal activity and cognitive deficits in AD.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
