MER5101, a novel Aβ1-15:DT conjugate vaccine, generates a robust anti-Aβ antibody response and attenuates Aβ pathology and cognitive deficits in APPswe/PS1ΔE9 transgenic mice
- PMID: 23595760
- PMCID: PMC3675782
- DOI: 10.1523/JNEUROSCI.5924-12.2013
MER5101, a novel Aβ1-15:DT conjugate vaccine, generates a robust anti-Aβ antibody response and attenuates Aβ pathology and cognitive deficits in APPswe/PS1ΔE9 transgenic mice
Abstract
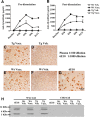
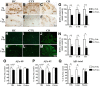
Active amyloid-β (Aβ) immunotherapy is under investigation to prevent or treat early Alzheimer's disease (AD). In 2002, a Phase II clinical trial (AN1792) was halted due to meningoencephalitis in ∼6% of the AD patients, possibly caused by a T-cell-mediated immunological response. Thus, generating a vaccine that safely generates high anti-Aβ antibody levels in the elderly is required. In this study, MER5101, a novel conjugate of Aβ1-15 peptide (a B-cell epitope fragment) conjugated to an immunogenic carrier protein, diphtheria toxoid (DT), and formulated in a nanoparticular emulsion-based adjuvant, was administered to 10-month-old APPswe/PS1ΔE9 transgenic (Tg) and wild-type (Wt) mice. High anti-Aβ antibody levels were observed in both vaccinated APPswe/PS1ΔE9 Tg and Wt mice. Antibody isotypes were mainly IgG1 and IgG2b, suggesting a Th2-biased response. Restimulation of splenocytes with the Aβ1-15:DT conjugate resulted in a strong proliferative response, whereas proliferation was absent after restimulation with Aβ1-15 or Aβ1-40/42 peptides, indicating a cellular immune response against DT while avoiding an Aβ-specific T-cell response. Moreover, significant reductions in cerebral Aβ plaque burden, accompanied by attenuated microglial activation and increased synaptic density, were observed in MER5101-vaccinated APPswe/PS1ΔE9 Tg mice compared with Tg adjuvant controls. Last, MER5101-immunized APPswe/PS1ΔE9 Tg mice showed improvement of cognitive deficits in both contextual fear conditioning and the Morris water maze. Our novel, highly immunogenic Aβ conjugate vaccine, MER5101, shows promise for improving Aβ vaccine safety and efficacy and therefore, may be useful for preventing and/or treating early AD.
Figures

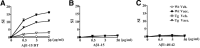



References
-
- Agadjanyan MG, Ghochikyan A, Petrushina I, Vasilevko V, Movsesyan N, Mkrtichyan M, Saing T, Cribbs DH. Prototype Alzheimer's disease vaccine using the immunodominant B cell epitope from beta-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. J Immunol. 2005;174:1580–1586. - PubMed
-
- Ajani JA, Hecht JR, Ho L, Baker J, Oortgiesen M, Eduljee A, Michaeli D. An open-label, multinational, multicenter study of G17DT vaccination combined with cisplatin and 5-fluorouracil in patients with untreated, advanced gastric or gastroesophageal cancer: the GC4 study. Cancer. 2006;106:1908–1916. doi: 10.1002/cncr.21814. - DOI - PubMed
-
- Asuni AA, Boutajangout A, Scholtzova H, Knudsen E, Li YS, Quartermain D, Frangione B, Wisniewski T, Sigurdsson EM. Vaccination of Alzheimer's model mice with Abeta derivative in alum adjuvant reduces Abeta burden without microhemorrhages. Eur J Neurosci. 2006;24:2530–2542. doi: 10.1111/j.1460-9568.2006.05149.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
