Molecular mechanism of constitutive endocytosis of Acid-sensing ion channel 1a and its protective function in acidosis-induced neuronal death
- PMID: 23595764
- PMCID: PMC4201763
- DOI: 10.1523/JNEUROSCI.5206-12.2013
Molecular mechanism of constitutive endocytosis of Acid-sensing ion channel 1a and its protective function in acidosis-induced neuronal death
Abstract
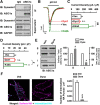
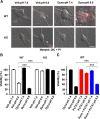
Acid-sensing ion channels (ASICs) are proton-gated cation channels widely expressed in the peripheral and CNSs, which critically contribute to a variety of pathophysiological conditions that involve tissue acidosis, such as ischemic stroke and epileptic seizures. However, the trafficking mechanisms of ASICs and the related proteins remain largely unknown. Here, we demonstrate that ASIC1a, the main ASIC subunit in the brain, undergoes constitutive endocytosis in a clathrin- and dynamin-dependent manner in both mouse cortical neurons and heterologous cell cultures. The endocytosis of ASIC1a was inhibited by either the small molecular inhibitor tyrphostin A23 or knockdown of the core subunit of adaptor protein 2 (AP2) μ2 using RNA interference, supporting a clathrin-dependent endocytosis of ASIC1a. In addition, the internalization of ASIC1a was blocked by dominant-negative dynamin1 mutation K44A and the small molecular inhibitor dynasore, suggesting that it is also dynamin-dependent. We show that the membrane-proximal residues (465)LCRRG(469) at the cytoplasmic C terminus of ASIC1a are critical for interaction with the endogenous adaptor protein complex and inhibition of ASIC1a internalization strongly exacerbated acidosis-induced death of cortical neurons from wild-type but not ASIC1a knock-out mice. Together, these results reveal the molecular mechanism of ASIC1a internalization and suggest the importance of endocytic pathway in functional regulation of ASIC1a channels as well as neuronal damages mediated by these channels during neurodegeneration.
Figures






Similar articles
-
Extracellular spermine exacerbates ischemic neuronal injury through sensitization of ASIC1a channels to extracellular acidosis.J Neurosci. 2011 Feb 9;31(6):2101-12. doi: 10.1523/JNEUROSCI.4351-10.2011. J Neurosci. 2011. PMID: 21307247 Free PMC article.
-
Cholestane-3β,5α,6β-Triol Inhibits Acid-Sensing Ion Channels and Reduces Acidosis-Mediated Ischemic Brain Injury.Stroke. 2024 Jun;55(6):1660-1671. doi: 10.1161/STROKEAHA.124.046963. Epub 2024 Apr 25. Stroke. 2024. PMID: 38660789 Free PMC article.
-
Acid Sensing Ion Channels (ASICs) in NS20Y cells - potential role in neuronal differentiation.Mol Brain. 2016 Jun 24;9(1):68. doi: 10.1186/s13041-016-0249-8. Mol Brain. 2016. PMID: 27342076 Free PMC article.
-
Acid-sensing ion channels: trafficking and pathophysiology.Channels (Austin). 2014;8(6):481-7. doi: 10.4161/19336950.2014.958382. Channels (Austin). 2014. PMID: 25483283 Free PMC article. Review.
-
Acid sensing ion channels--novel therapeutic targets for ischemic brain injury.Front Biosci. 2007 Jan 1;12:1376-86. doi: 10.2741/2154. Front Biosci. 2007. PMID: 17127388 Review.
Cited by
-
Acid-sensing ion channels: dual function proteins for chemo-sensing and mechano-sensing.J Biomed Sci. 2018 May 24;25(1):46. doi: 10.1186/s12929-018-0448-y. J Biomed Sci. 2018. PMID: 29793480 Free PMC article. Review.
-
Altered Expression Pattern of Acid-Sensing Ion Channel Isoforms in Piriform Cortex After Seizures.Mol Neurobiol. 2016 Apr;53(3):1782-1793. doi: 10.1007/s12035-015-9130-5. Epub 2015 Mar 7. Mol Neurobiol. 2016. PMID: 25744567
-
The Protein Tyrosine Kinase Inhibitor Tyrphostin 23 Strongly Accelerates Glycolytic Lactate Production in Cultured Primary Astrocytes.Neurochem Res. 2016 Oct;41(10):2607-2618. doi: 10.1007/s11064-016-1972-3. Epub 2016 Jun 9. Neurochem Res. 2016. PMID: 27278759
-
Acid-Sensing Ion Channels: Expression and Function in Resident and Infiltrating Immune Cells in the Central Nervous System.Front Cell Neurosci. 2021 Sep 17;15:738043. doi: 10.3389/fncel.2021.738043. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34602982 Free PMC article. Review.
-
Factors and Molecular Mechanisms Influencing the Protein Synthesis, Degradation and Membrane Trafficking of ASIC1a.Front Cell Dev Biol. 2020 Oct 23;8:596304. doi: 10.3389/fcell.2020.596304. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33195276 Free PMC article. Review.
References
-
- Banbury DN, Oakley JD, Sessions RB, Banting G. Tyrphostin A23 inhibits internalization of the transferrin receptor by perturbing the interaction between tyrosine motifs and the medium chain subunit of the AP-2 adaptor complex. J Biol Chem. 2003;278:12022–12028. doi: 10.1074/jbc.M211966200. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
