Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling
- PMID: 23595904
- PMCID: PMC4070868
- DOI: 10.1095/biolreprod.113.108555
Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling
Abstract
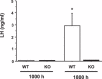
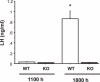
Kisspeptin stimulates gonadotropin-releasing hormone (GnRH) neurons via the kisspeptin receptor, Kiss1r. In rodents, estrogen-responsive kisspeptin neurons in the rostral hypothalamus have been postulated to mediate estrogen-induced positive feedback induction of the preovulatory luteinizing hormone (LH) surge. However, conflicting evidence exists regarding the ability of mice lacking Kiss1r to display LH surges in response to exogenous hormones. Whether the discrepancy reflects different mouse strains used and/or utilization of different surge-induction paradigms is unknown. Here, we tested multiple hormonal paradigms in one Kiss1r knockout (KO) model to see which paradigms, if any, could generate circadian-timed LH surges. Kiss1r KO and wild-type (WT) females were ovariectomized, given sex steroids in various modes, and assessed several days later for LH levels in the morning or evening (when surges occur). Serum LH levels were very low in all morning animals, regardless of genotype or hormonal paradigm. In each paradigm, virtually all WT females displayed clear LH surges in the evening, whereas none of the KO females demonstrated LH surges. The lack of LH surges in KO mice reflects a lack of GnRH secretion rather than diminished pituitary responsiveness from a lifetime lack of GnRH exposure because KO mice responded to GnRH priming with robust LH secretion. Moreover, high cfos-GnRH coexpression was detected in WT females in the evening, whereas low cfos-GnRH coexpression was present in KO females at all time points. Our findings conclusively demonstrate that WT females consistently display LH surges under multiple hormonal paradigms, whereas Kiss1r KO mice do not, indicating that kisspeptin-Kiss1r signaling is mandatory for GnRH/LH surge induction.
Keywords: GPR54; GnRH; Kiss1; Kiss1r; LH surge; estradiol; kisspeptin; positive feedback.
Figures




Similar articles
-
Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge.J Neurosci. 2008 Aug 27;28(35):8691-7. doi: 10.1523/JNEUROSCI.1775-08.2008. J Neurosci. 2008. PMID: 18753370 Free PMC article.
-
Progesterone Receptors in AVPV Kisspeptin Neurons Are Sufficient for Positive Feedback Induction of the LH Surge.Endocrinology. 2021 Nov 1;162(11):bqab161. doi: 10.1210/endocr/bqab161. Endocrinology. 2021. PMID: 34379733 Free PMC article.
-
Absent Progesterone Signaling in Kisspeptin Neurons Disrupts the LH Surge and Impairs Fertility in Female Mice.Endocrinology. 2015 Sep;156(9):3091-7. doi: 10.1210/en.2015-1300. Epub 2015 Jun 15. Endocrinology. 2015. PMID: 26076042 Free PMC article.
-
The role of kisspeptin and RFamide-related peptide-3 neurones in the circadian-timed preovulatory luteinising hormone surge.J Neuroendocrinol. 2012 Jan;24(1):131-43. doi: 10.1111/j.1365-2826.2011.02162.x. J Neuroendocrinol. 2012. PMID: 21592236 Free PMC article. Review.
-
Interaction between ovarian and adrenal steroids in the regulation of gonadotropin secretion.J Steroid Biochem Mol Biol. 1992 Mar;41(3-8):495-513. doi: 10.1016/0960-0760(92)90375-s. J Steroid Biochem Mol Biol. 1992. PMID: 1562521 Review.
Cited by
-
Estrogen Regulation of the Molecular Phenotype and Active Translatome of AVPV Kisspeptin Neurons.Endocrinology. 2021 Sep 1;162(9):bqab080. doi: 10.1210/endocr/bqab080. Endocrinology. 2021. PMID: 33856454 Free PMC article.
-
Metabolism and Energy Expenditure, But Not Feeding or Glucose Tolerance, Are Impaired in Young Kiss1r KO Female Mice.Endocrinology. 2016 Nov;157(11):4192-4199. doi: 10.1210/en.2016-1501. Epub 2016 Sep 20. Endocrinology. 2016. PMID: 27649089 Free PMC article.
-
Circadian Regulation of the Brain and Behavior: A Neuroendocrine Perspective.Curr Top Behav Neurosci. 2019;43:323-351. doi: 10.1007/7854_2019_115. Curr Top Behav Neurosci. 2019. PMID: 31586337 Free PMC article.
-
Rodent Models of Non-classical Progesterone Action Regulating Ovulation.Front Endocrinol (Lausanne). 2017 Jul 24;8:165. doi: 10.3389/fendo.2017.00165. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28790975 Free PMC article. Review.
-
Deletion of the homeodomain gene Six3 from kisspeptin neurons causes subfertility in female mice.Mol Cell Endocrinol. 2022 Apr 15;546:111577. doi: 10.1016/j.mce.2022.111577. Epub 2022 Feb 2. Mol Cell Endocrinol. 2022. PMID: 35121076 Free PMC article.
References
-
- Ojeda SR, Skinner MK. Puberty in the rat. Neill JD. (ed.), Knobil and Niell's Physiology of Reproduction, 3rd ed. San Diego: Elsevier; 2006: 2061 2126.
-
- Freeman ME. Neuroendocrine control of the ovarian cycle in the rat. : Neill JD. (ed.), Physiology of Reproduction, 3rd ed. San Diego: Elsevier; 2006: 2283 2326.
-
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 2006; 52: 271 280. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

