Gelsolin expression increases β1 -integrin affinity and L1210 cell adhesion
- PMID: 23595955
- PMCID: PMC4074084
- DOI: 10.1002/cm.21112
Gelsolin expression increases β1 -integrin affinity and L1210 cell adhesion
Abstract
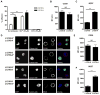
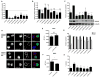
Integrins are functionally regulated by "inside-out" signaling, in that stimulus-induced signaling pathways act on the intracellular integrin tail to regulate the activity of the receptor on the outside. Both a change in conformation (affinity) and clustering (avidity/valency) of the receptors occurs, but the mechanisms that regulate inside out signaling are not completely understood. Previously, we identified gelsolin in a proteomics screen to identify proteins involved in inside-out control of integrins using the lymphocytic leukemia cell line L1210. Furthermore, we showed that gelsolin was involved in affinity regulation of β1 -integrins in the leukemic cell line U937. Here, we examined how gelsolin regulates β1 -integrin affinity in the leukemia cell line L1210. We show that gelsolin is mainly expressed at the cell membrane and is present near β1 -integrins. The role for actin polymerization in integrin affinity regulation was examined using the actin modulating agent jasplakinolide, which decreased β1 -integrin affinity. Similarly, knock-down of gelsolin in L1210 cells also decreased β1 -integrin affinity and cell adhesion to collagen. These data suggest that increased actin polymerization through gelsolin regulates β1 -integrin affinity and cell adhesion.
Keywords: attachment; cell membrane; cytoskeleton; gelsolin; migration.
Copyright © 2013 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

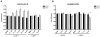
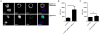
Similar articles
-
A 2D-DIGE approach to identify proteins involved in inside-out control of integrins.J Proteome Res. 2009 Aug;8(8):3824-33. doi: 10.1021/pr8010815. J Proteome Res. 2009. PMID: 19505086
-
Neutrophil integrin affinity regulation in adhesion, migration, and bacterial clearance.Cell Adh Migr. 2013 Nov-Dec;7(6):476-81. doi: 10.4161/cam.27293. Epub 2013 Dec 2. Cell Adh Migr. 2013. PMID: 24430200 Free PMC article. Review.
-
Loss of ADAM9 expression impairs β1 integrin endocytosis, focal adhesion formation and cancer cell migration.J Cell Sci. 2018 Jan 4;131(1):jcs205393. doi: 10.1242/jcs.205393. J Cell Sci. 2018. PMID: 29142101
-
PRL-3/PTP4A3 phosphatase regulates integrin β1 in adhesion structures during migration of human ocular melanoma cells.Exp Cell Res. 2017 Apr 15;353(2):88-99. doi: 10.1016/j.yexcr.2017.03.012. Epub 2017 Mar 8. Exp Cell Res. 2017. PMID: 28284838
-
Mechanisms of integrin activation and trafficking.Curr Opin Cell Biol. 2011 Oct;23(5):607-14. doi: 10.1016/j.ceb.2011.08.005. Epub 2011 Sep 14. Curr Opin Cell Biol. 2011. PMID: 21924601 Review.
Cited by
-
Skin melanoma cells produce diverse gelsolin (GSN) isoforms, which play non-redundant roles in cells' proliferation and motility.Cancer Cell Int. 2025 Jul 1;25(1):239. doi: 10.1186/s12935-025-03876-x. Cancer Cell Int. 2025. PMID: 40597347 Free PMC article.
-
Gelsolin-mediated activation of PI3K/Akt pathway is crucial for hepatocyte growth factor-induced cell scattering in gastric carcinoma.Oncotarget. 2016 May 3;7(18):25391-407. doi: 10.18632/oncotarget.8603. Oncotarget. 2016. PMID: 27058427 Free PMC article.
-
Gelsolin Contributes to the Motility of A375 Melanoma Cells and This Activity Is Mediated by the Fibrous Extracellular Matrix Protein Profile.Cells. 2021 Jul 21;10(8):1848. doi: 10.3390/cells10081848. Cells. 2021. PMID: 34440617 Free PMC article.
-
MFG-E8-derived peptide attenuates adhesion and migration of immune cells to endothelial cells.J Leukoc Biol. 2017 May;101(5):1201-1209. doi: 10.1189/jlb.3A0416-184RR. Epub 2017 Jan 17. J Leukoc Biol. 2017. PMID: 28096298 Free PMC article.
-
Tumor-associated and disease-associated autoantibody repertoires in healthy colostrum and maternal and newborn cord sera.J Immunol. 2015 Jun 1;194(11):5272-81. doi: 10.4049/jimmunol.1402771. Epub 2015 Apr 27. J Immunol. 2015. PMID: 25917091 Free PMC article.
References
-
- Bennett JS, Zigmond S, Vilaire G, Cunningham ME, Bednar B. The platelet cytoskeleton regulates the affinity of the integrin alpha(IIb)beta(3) for fibrinogen. J Biol Chem. 1999;274(36):25301–7. - PubMed
-
- Burtnick LD, Koepf EK, Grimes J, Jones EY, Stuart DI, McLaughlin PJ, Robinson RC. The crystal structure of plasma gelsolin: implications for actin severing, capping, and nucleation. Cell. 1997;90(4):661–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

