Targeted deletion of growth hormone (GH) receptor in macrophage reveals novel osteopontin-mediated effects of GH on glucose homeostasis and insulin sensitivity in diet-induced obesity
- PMID: 23595986
- PMCID: PMC3668731
- DOI: 10.1074/jbc.M113.460212
Targeted deletion of growth hormone (GH) receptor in macrophage reveals novel osteopontin-mediated effects of GH on glucose homeostasis and insulin sensitivity in diet-induced obesity
Abstract
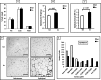
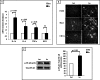
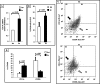
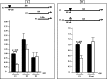
We investigated GH action on macrophage (MΦ) by creating a MΦ-specific GH receptor-null mouse model (MacGHR KO). On a normal diet (10% fat), MacGHR KO and littermate controls exhibited similar growth profiles and glucose excursions on intraperitoneal glucose (ipGTT) and insulin tolerance (ITT) tests. However, when challenged with high fat diet (HFD, 45% fat) for 18 weeks, MacGHR KO mice exhibited impaired ipGTT and ITT compared with controls. In MacGHR KO, adipose-tissue (AT) MΦ abundance was increased with skewing toward M1 polarization. Expression of pro-inflammatory cytokines (IL1β, TNF-α, IL6, and osteopontin (OPN)) were increased in MacGHR KO AT stromal vascular fraction (SVF). In MacGHR KO AT, crown-like-structures were increased with decreased insulin-dependent Akt phosphorylation. The abundance of phosphorylated NF-κB and of OPN was increased in SVF and bone-marrow-derived MΦ in MacGHR KO. GH, acting via an NF-κB site in the distal OPN promoter, inhibited the OPN promoter. Thus in diet-induced obesity (DIO), lack of GH action on the MΦ exerts an unexpected deleterious effect on glucose homeostasis by accentuating AT inflammation and NF-κB-dependent activation of OPN expression. These novel results in mice support the possibility that administration of GH could have salutary effects on DIO-associated chronic inflammation and insulin resistance in humans.
Keywords: Adipose Tissue; Growth Hormone; Inflammation; Macrophages; Obesity.
Figures




References
-
- Newbern D., Freemark M. (2011) Placental hormones and the control of maternal metabolism and fetal growth. Curr. Opin. Endocrinol. Diabetes Obes. 18, 409–416 - PubMed
-
- Bloch C. A., Clemons P., Sperling M. A. (1987) Puberty decreases insulin sensitivity. J. Pediatr. 110, 481–487 - PubMed
-
- Clayton P. E., Cowell C. T. (2000) Safety issues in children and adolescents during growth hormone therapy–a review. Growth Horm IGF Res. 10, 306–317 - PubMed
-
- Møller N., Jørgensen J. O. (2009) Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 30, 152–177 - PubMed
-
- Johansen T., Laurino C., Barreca A., Malmlöf K. (2005) Reduction of adiposity with prolonged growth hormone treatment in old obese rats: effects on glucose handling and early insulin signaling. Growth Horm IGF Res. 15, 55–63 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

