Regulation of a dynamic interaction between two microtubule-binding proteins, EB1 and TIP150, by the mitotic p300/CBP-associated factor (PCAF) orchestrates kinetochore microtubule plasticity and chromosome stability during mitosis
- PMID: 23595990
- PMCID: PMC3668735
- DOI: 10.1074/jbc.M112.448886
Regulation of a dynamic interaction between two microtubule-binding proteins, EB1 and TIP150, by the mitotic p300/CBP-associated factor (PCAF) orchestrates kinetochore microtubule plasticity and chromosome stability during mitosis
Abstract
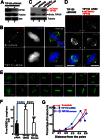
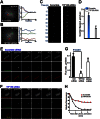
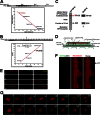
The microtubule cytoskeleton network orchestrates cellular dynamics and chromosome stability in mitosis. Although tubulin acetylation is essential for cellular plasticity, it has remained elusive how kinetochore microtubule plus-end dynamics are regulated by p300/CBP-associated factor (PCAF) acetylation in mitosis. Here, we demonstrate that the plus-end tracking protein, TIP150, regulates dynamic kinetochore-microtubule attachments by promoting the stability of spindle microtubule plus-ends. Suppression of TIP150 by siRNA results in metaphase alignment delays and perturbations in chromosome biorientation. TIP150 is a tetramer that binds an end-binding protein (EB1) dimer through the C-terminal domains, and overexpression of the C-terminal TIP150 or disruption of the TIP150-EB1 interface by a membrane-permeable peptide perturbs chromosome segregation. Acetylation of EB1-PCAF regulates the TIP150 interaction, and persistent acetylation perturbs EB1-TIP150 interaction and accurate metaphase alignment, resulting in spindle checkpoint activation. Suppression of the mitotic checkpoint serine/threonine protein kinase, BubR1, overrides mitotic arrest induced by impaired EB1-TIP150 interaction, but cells exhibit whole chromosome aneuploidy. Thus, the results identify a mechanism by which the TIP150-EB1 interaction governs kinetochore microtubule plus-end plasticity and establish that the temporal control of the TIP150-EB1 interaction by PCAF acetylation ensures chromosome stability in mitosis.
Keywords: Acetyl Coenzyme A; Cell Cycle; Genomic Instability; Mitosis; Mitotic Spindle.
Figures




References
-
- Cleveland D. W., Mao Y., Sullivan K. F. (2003) Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell 112, 407–421 - PubMed
-
- Gordon D. J., Resio B., Pellman D. (2012) Causes and consequences of aneuploidy in cancers. Nat. Rev. Genet. 13, 189–203 - PubMed
-
- Cheeseman I. M., Desai A. (2008) Molecular architecture of the kinetochore-microtubule interface. Nat. Rev. Mol. Cell Biol. 9, 33–46 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

