Extracellular nucleotides inhibit oxalate transport by human intestinal Caco-2-BBe cells through PKC-δ activation
- PMID: 23596171
- PMCID: PMC3725516
- DOI: 10.1152/ajpcell.00339.2012
Extracellular nucleotides inhibit oxalate transport by human intestinal Caco-2-BBe cells through PKC-δ activation
Abstract
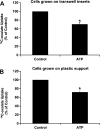
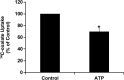
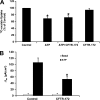
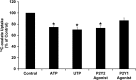
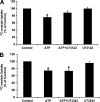
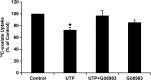
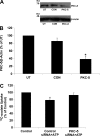
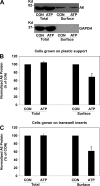
Nephrolithiasis remains a major health problem in Western countries. Seventy to 80% of kidney stones are composed of calcium oxalate, and small changes in urinary oxalate affect risk of kidney stone formation. Intestinal oxalate secretion mediated by the anion exchanger SLC26A6 plays an essential role in preventing hyperoxaluria and calcium oxalate nephrolithiasis, indicating that understanding the mechanisms regulating intestinal oxalate transport is critical for management of hyperoxaluria. Purinergic signaling modulates several intestinal processes through pathways including PKC activation, which we previously found to inhibit Slc26a6 activity in mouse duodenal tissue. We therefore examined whether purinergic stimulation with ATP and UTP affects oxalate transport by human intestinal Caco-2-BBe (C2) cells. We measured [¹⁴C]oxalate uptake in the presence of an outward Cl⁻ gradient as an assay of Cl⁻/oxalate exchange activity, ≥50% of which is mediated by SLC26A6. We found that ATP and UTP significantly inhibited oxalate transport by C2 cells, an effect blocked by the PKC inhibitor Gö-6983. Utilizing pharmacological agonists and antagonists, as well as PKC-δ knockdown studies, we observed that ATP inhibits oxalate transport through the P2Y₂ receptor, PLC, and PKC-δ. Biotinylation studies showed that ATP inhibits oxalate transport by lowering SLC26A6 surface expression. These findings are of potential relevance to pathophysiology of inflammatory bowel disease-associated hyperoxaluria, where supraphysiological levels of ATP/UTP are expected and overexpression of the P2Y₂ receptor has been reported. We conclude that ATP and UTP inhibit oxalate transport by lowering SLC26A6 surface expression in C2 cells through signaling pathways including the P2Y₂ purinergic receptor, PLC, and PKC-δ.
Keywords: P2Y2 purinergic receptor; PKC-δ; SLC26A6; phospholipase C.
Figures

References
-
- Ao M, Venkatasubramanian J, Boonkaewwan C, Ganesan N, Syed A, Benya RV, Rao MC. Lubiprostone activates Cl− secretion via cAMP signaling and increases membrane CFTR in the human colon carcinoma cell line, T84. Dig Dis Sci 56: 339– 351, 2011 - PubMed
-
- Arpin M, Blair L, Coudrier E, Dudouet B, Finidori J, Carcia A, Huet C, Pringault E, Robine S, Sahuguillo-Merino C, et al. Villin, a specific marker for some epithelia specialized in transport, to study the differentiation of intestinal and kidney cells in vivo and in a human colon adenocarcinoma line HT29 in culture. Mol Aspects Med 10: 257– 272, 1988 - PubMed
-
- Asplin JR. Hyperoxaluric calcium nephrolithiasis. Endocrinol Metab Clin North Am 31: 927– 949, 2002 - PubMed
-
- Athman R, Louvard D, Robine S. The epithelial cell cytoskeleton and intracellular trafficking. III. How is villin involved in the actin cytoskeleton dynamics in intestinal cells? Am J Physiol Gastrointest Liver Physiol 283: G496– G502, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

