Attenuated and replication-competent vaccinia virus strains M65 and M101 with distinct biology and immunogenicity as potential vaccine candidates against pathogens
- PMID: 23596295
- PMCID: PMC3676094
- DOI: 10.1128/JVI.03013-12
Attenuated and replication-competent vaccinia virus strains M65 and M101 with distinct biology and immunogenicity as potential vaccine candidates against pathogens
Abstract
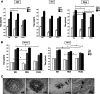
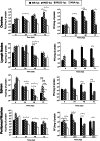
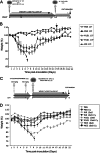
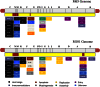
Replication-competent poxvirus vectors with an attenuation phenotype and with a high immunogenic capacity of the foreign expressed antigen are being pursued as novel vaccine vectors against different pathogens. In this investigation, we have examined the replication and immunogenic characteristics of two vaccinia virus (VACV) mutants, M65 and M101. These mutants were generated after 65 and 101 serial passages of persistently infected Friend erythroleukemia (FEL) cells. In cultured cells of different origins, the mutants are replication competent and have growth kinetics similar to or slightly reduced in comparison with those of the parental Western Reserve (WR) virus strain. In normal and immune-suppressed infected mice, the mutants showed different levels of attenuation and pathogenicity in comparison with WR and modified vaccinia Ankara (MVA) strains. Wide genome analysis after deep sequencing revealed selected genomic deletions and mutations in a number of viral open reading frames (ORFs). Mice immunized in a DNA prime/mutant boost regimen with viral vectors expressing the LACK (Leishmania homologue for receptors of activated C kinase) antigen of Leishmania infantum showed protection or a delay in the onset of cutaneous leishmaniasis. Protection was similar to that triggered by MVA-LACK. In immunized mice, both polyfunctional CD4(+) and CD8(+) T cells with an effector memory phenotype were activated by the two mutants, but the DNA-LACK/M65-LACK protocol preferentially induced CD4(+) whereas DNA-LACK/M101-LACK preferentially induced CD8(+) T cell responses. Altogether, our findings showed the adaptive changes of the WR genome during long-term virus-host cell interaction and how the replication competency of M65 and M101 mutants confers distinct biological properties and immunogenicity in mice compared to those of the MVA strain. These mutants could have applicability for understanding VACV biology and as potential vaccine vectors against pathogens and tumors.
Figures



References
-
- Gómez CE, Najera JL, Krupa M, Perdiguero B, Esteban M. 2011. MVA and NYVAC as vaccines against emergent infectious diseases and cancer. Curr. Gene Ther. 11:189–217 - PubMed
-
- Gómez CE, Najera JL, Krupa M, Esteban M. 2008. The poxvirus vectors MVA and NYVAC as gene delivery systems for vaccination against infectious diseases and cancer. Curr. Gene Ther. 8:97–120 - PubMed
-
- Esteban M. 2009. Attenuated poxvirus vectors MVA and NYVAC as promising vaccine candidates against HIV/AIDS. Hum. Vaccin. 5:867–871 - PubMed
-
- Pantaleo G, Esteban M, Jacobs B, Tartaglia J. 2010. Poxvirus vector-based HIV vaccines. Curr. Opin. HIV AIDS 5:391–396 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

