Potential of herpesvirus saimiri-based vectors to reprogram a somatic Ewing's sarcoma family tumor cell line
- PMID: 23596304
- PMCID: PMC3676103
- DOI: 10.1128/JVI.03147-12
Potential of herpesvirus saimiri-based vectors to reprogram a somatic Ewing's sarcoma family tumor cell line
Abstract
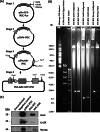
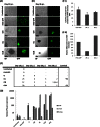
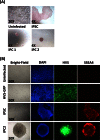
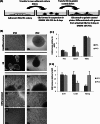
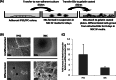
Herpesvirus saimiri (HVS) infects a range of human cell types with high efficiency. Upon infection, the viral genome can persist as high-copy-number, circular, nonintegrated episomes that segregate to progeny cells upon division. This allows HVS-based vectors to stably transduce a dividing cell population and provide sustained transgene expression in vitro and in vivo. Moreover, the HVS episome is able to persist and provide prolonged transgene expression during in vitro differentiation of mouse and human hemopoietic progenitor cells. Together, these properties are advantageous for induced pluripotent stem cell (iPSC) technology, whereby stem cell-like cells are generated from adult somatic cells by exogenous expression of specific reprogramming factors. Here we assess the potential of HVS-based vectors for the generation of induced pluripotent cancer stem-like cells (iPCs). We demonstrate that HVS-based exogenous delivery of Oct4, Nanog, and Lin28 can reprogram the Ewing's sarcoma family tumor cell line A673 to produce stem cell-like colonies that can grow under feeder-free stem cell culture conditions. Further analysis of the HVS-derived putative iPCs showed some degree of reprogramming into a stem cell-like state. Specifically, the putative iPCs had a number of embryonic stem cell characteristics, staining positive for alkaline phosphatase and SSEA4, in addition to expressing elevated levels of pluripotent marker genes involved in proliferation and self-renewal. However, differentiation trials suggest that although the HVS-derived putative iPCs are capable of differentiation toward the ectodermal lineage, they do not exhibit pluripotency. Therefore, they are hereby termed induced multipotent cancer cells.
Figures

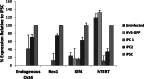
Similar articles
-
Herpesvirus saimiri-based vector biodistribution using noninvasive optical imaging.Gene Ther. 2005 Oct;12(19):1465-76. doi: 10.1038/sj.gt.3302543. Gene Ther. 2005. PMID: 15889136
-
Assessment of infectivity using a Herpesvirus saimiri (HVS) recombinant that expresses HVS-GFP.Cold Spring Harb Protoc. 2011 Dec 1;2011(12):1520-3. doi: 10.1101/pdb.prot066951. Cold Spring Harb Protoc. 2011. PMID: 22135663
-
Production of recombinant Herpesvirus saimiri-based vectors.Cold Spring Harb Protoc. 2011 Dec 1;2011(12):1515-9. doi: 10.1101/pdb.prot066944. Cold Spring Harb Protoc. 2011. PMID: 22135662
-
Herpesvirus saimiri-based gene delivery vectors.Curr Gene Ther. 2006 Feb;6(1):1-15. doi: 10.2174/156652306775515529. Curr Gene Ther. 2006. PMID: 16475942 Review.
-
Development of herpesvirus-based episomally maintained gene delivery vectors.Expert Opin Biol Ther. 2004 Apr;4(4):493-505. doi: 10.1517/14712598.4.4.493. Expert Opin Biol Ther. 2004. PMID: 15102599 Review.
Cited by
-
Epigenetic reprogramming and re-differentiation of a Ewing sarcoma cell line.Front Cell Dev Biol. 2015 Mar 9;3:15. doi: 10.3389/fcell.2015.00015. eCollection 2015. Front Cell Dev Biol. 2015. PMID: 25806369 Free PMC article.
References
-
- Takahashi K, Yamanaka S. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676 - PubMed
-
- Grskovic M, Javaherian A, Strulovici B, Daley GQ. 2011. Induced pluripotent stem cells—opportunities for disease modelling and drug discovery. Nat. Rev. Drug Discov. 10:915–929 - PubMed
-
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. 2007. Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920 - PubMed
-
- Kim JB, Greber B, Arauzo-Bravo MJ, Meyer J, Park KI, Zaehres H, Scholer HR. 2009. Direct reprogramming of human neural stem cells by OCT4. Nature 461:649–653 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

