Identification of candidate genes encoding an LDL-C QTL in baboons
- PMID: 23596326
- PMCID: PMC3679381
- DOI: 10.1194/jlr.M032649
Identification of candidate genes encoding an LDL-C QTL in baboons
Erratum in
- J Lipid Res. 2013 Aug;54(8):2291. Hafizi, Sassan [added]
Abstract
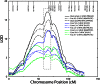
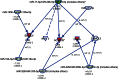
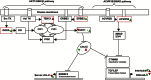
Cardiovascular disease (CVD) is the leading cause of death in developed countries, and dyslipidemia is a major risk factor for CVD. We previously identified a cluster of quantitative trait loci (QTL) on baboon chromosome 11 for multiple, related quantitative traits for serum LDL-cholesterol (LDL-C). Here we report differentially regulated hepatic genes encoding an LDL-C QTL that influences LDL-C levels in baboons. We performed hepatic whole-genome expression profiling for LDL-C-discordant baboons fed a high-cholesterol, high-fat (HCHF) diet for seven weeks. We detected expression of 117 genes within the QTL 2-LOD support interval. Three genes were differentially expressed in low LDL-C responders and 8 in high LDL-C responders in response to a HCHF diet. Seven genes (ACVR1B, CALCOCO1, DGKA, ERBB3, KRT73, MYL6B, TENC1) showed discordant expression between low and high LDL-C responders. To prioritize candidate genes, we integrated miRNA and mRNA expression profiles using network tools and found that four candidates (ACVR1B, DGKA, ERBB3, TENC1) were miRNA targets and that the miRNAs were inversely expressed to the target genes. Candidate gene expression was validated using QRT-PCR and Western blotting. This study reveals candidate genes that influence variation in LDL-C in baboons and potential genetic mechanisms for further investigation.
Keywords: cardiovascular disease; diet-responsive liver gene expression; dyslipidemia; low density lipoprotein-cholesterol.
Figures
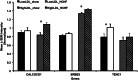

References
-
- Ross R. 1993. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 362: 801–809 - PubMed
-
- McGill H. C., Jr, McMahan C. A., Herderick E. E., Tracy R. E., Malcom G. T., Zieske A. W., Strong J. P. 2000. Effects of coronary heart disease risk factors on atherosclerosis of selected regions of the aorta and right coronary artery. PDAY Research Group. Pathobiological Determinants of Atherosclerosis in Youth. Arterioscler. Thromb. Vasc. Biol. 20: 836–845 - PubMed
-
- Kushwaha R. S., McGill H. C., Jr 1998. Diet, plasma lipoproteins and experimental atherosclerosis in baboons (Papio sp.). Hum. Reprod. Update. 4: 420–429 - PubMed
-
- Libby P. 2006. Atherosclerosis: disease biology affecting the coronary vasculature. Am. J. Cardiol. 98: 3Q–9Q - PubMed
-
- Barter P. J., Rye K. A. 1996. Molecular mechanisms of reverse cholesterol transport. Curr. Opin. Lipidol. 7: 82–87 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

