The Nav1.9 channel regulates colonic motility in mice
- PMID: 23596386
- PMCID: PMC3625748
- DOI: 10.3389/fnins.2013.00058
The Nav1.9 channel regulates colonic motility in mice
Abstract
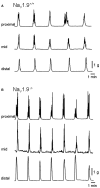
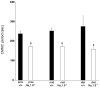
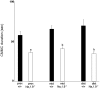
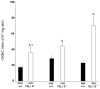
The colonic migrating motor complex (CMMC) is a major pattern of motility that is entirely generated and organized by the enteric nervous system. We have previously demonstrated that the Nav1.9 channel underlies a tetrodotoxin-resistant sodium current which modulates the excitability of enteric neurons. The aim of this study was to observe the effect of loss of the Nav1.9 channel in enteric neurons on mouse colonic motility in vitro. The mechanical activity of the circular muscle was simultaneously recorded from three sites, namely, proximal, mid- and distal, along the whole colon of male, age-matched wild-type and Nav1.9 null mice. Spontaneous CMMCs were observed in all preparations. The mean frequency of CMMCs was significantly higher in the Nav1.9 null mice (one every 2.87 ± 0.1 min compared to one every 3.96 ± 0.23 min in the wild type). The mean duration of CMMCs was shorter and the mean area-under-contraction was larger in the Nav1.9 null mice compared to the wild type. In addition, CMMCs propagated preferentially in an aboral direction in the Nav1.9 null mice. Our study demonstrates that CMMCs do occur in mice lacking the Nav1.9 channel, but their characteristics are significantly different from controls. Up to now, the Nav1.9 channel was mainly associated with nociceptive neurons and involved in their hyperexcitability after inflammation. Our result shows for the first time a role for the Nav1.9 channel in a complex colonic motor pattern.
Keywords: Nav1.9 channel; colon; enteric; migrating motor complex; neuron.
Figures

References
-
- Bertrand P. P., Kunze W. A., Bornstein J. C., Furness J. B., Smith M. L. (1997). Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. Am. J. Physiol. 273, G422–G435 - PubMed
-
- Bertrand P. P., Kunze W. A., Furness J. B., Bornstein J. C. (2000). The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience 101, 459–469 10.1016/S0306-4522(00)00363-8 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

