Multifunctional role of astrocytes as gatekeepers of neuronal energy supply
- PMID: 23596393
- PMCID: PMC3622037
- DOI: 10.3389/fncel.2013.00038
Multifunctional role of astrocytes as gatekeepers of neuronal energy supply
Abstract
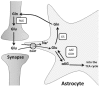
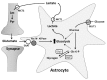
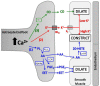
Dynamic adjustments to neuronal energy supply in response to synaptic activity are critical for neuronal function. Glial cells known as astrocytes have processes that ensheath most central synapses and express G-protein-coupled neurotransmitter receptors and transporters that respond to neuronal activity. Astrocytes also release substrates for neuronal oxidative phosphorylation and have processes that terminate on the surface of brain arterioles and can influence vascular smooth muscle tone and local blood flow. Membrane receptor or transporter-mediated effects of glutamate represent a convergence point of astrocyte influence on neuronal bioenergetics. Astrocytic glutamate uptake drives glycolysis and subsequent shuttling of lactate from astrocytes to neurons for oxidative metabolism. Astrocytes also convert synaptically reclaimed glutamate to glutamine, which is returned to neurons for glutamate salvage or oxidation. Finally, astrocytes store brain energy currency in the form of glycogen, which can be mobilized to produce lactate for neuronal oxidative phosphorylation in response to glutamatergic neurotransmission. These mechanisms couple synaptically driven astrocytic responses to glutamate with release of energy substrates back to neurons to match demand with supply. In addition, astrocytes directly influence the tone of penetrating brain arterioles in response to glutamatergic neurotransmission, coordinating dynamic regulation of local blood flow. We will describe the role of astrocytes in neurometabolic and neurovascular coupling in detail and discuss, in turn, how astrocyte dysfunction may contribute to neuronal bioenergetic deficit and neurodegeneration. Understanding the role of astrocytes as a hub for neurometabolic and neurovascular coupling mechanisms is a critical underpinning for therapeutic development in a broad range of neurodegenerative disorders characterized by chronic generalized brain ischemia and brain microvascular dysfunction.
Keywords: Alzheimer's disease; astrocytes; brain oxidative metabolism; epilepsy; glutamate-glutamine shuttle; ischemia; neurovascular coupling.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

