δ-Opioid receptor activation modified microRNA expression in the rat kidney under prolonged hypoxia
- PMID: 23596515
- PMCID: PMC3626642
- DOI: 10.1371/journal.pone.0061080
δ-Opioid receptor activation modified microRNA expression in the rat kidney under prolonged hypoxia
Abstract
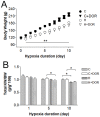
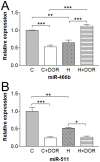
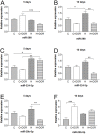
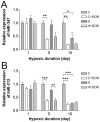
Hypoxic/ischemic injury to kidney is a frequently encountered clinical problem with limited therapeutic options. Since microRNAs are differentially involved in hypoxic/ischemic events and δ-opioid receptor (DOR) activation is known to protect against hypoxic/ischemic injury, we speculated on the involvement of DOR activation in altering the microRNA (miRNA) expression in kidney under hypoxic condition. We selected 31 miRNAs based on microarray data for quantitative PCR analysis. Among them, 14 miRNAs were significantly altered after prolonged hypoxia, DOR activation or a combination of both. We found that 1) DOR activation alters miRNA expression profiles in normoxic conditions; 2) hypoxia differentially alters miRNA expression depending on the duration of hypoxia; and 3) DOR activation can modify hypoxia-induced changes in miRNA expression. For example, 10-day hypoxia reduced the level of miR-212 by over 70%, while DOR activation could mimic such reduction even in normoxic kidney. In contrast, the same stress increased miR-29a by >100%, which was reversed following DOR activation. These first data suggest that hypoxia comprehensively modifies the miRNA profile within the kidney, which can be mimicked or modified by DOR activation. Ascertaining the targeted pathways that regulate the diverse cellular and molecular functions of miRNA may provide new insights into potential therapies for hypoxic/ischemic injury of the kidney.
Conflict of interest statement
Figures




References
-
- Nangaku M, Eckardt KU (2007) Hypoxia and the HIF system in kidney disease. J Mol Med 85: 1325–1330. - PubMed
-
- Heyman SN, Khamaisi M, Rosen S, Rosenberger C (2008) Renal Parenchymal Hypoxia, Hypoxia Response and the Progression of Chronic Kidney Disease. Am J Nephrol 28: 998–1006. - PubMed
-
- Martínez-Ballarín E, Pié J, Martínez-Berganza A (1986) Effects of chronic hypoxia on kidney function. Rev Esp Fisiol 42: 319–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

