A novel cell lysis approach reveals that caspase-2 rapidly translocates from the nucleus to the cytoplasm in response to apoptotic stimuli
- PMID: 23596516
- PMCID: PMC3626589
- DOI: 10.1371/journal.pone.0061085
A novel cell lysis approach reveals that caspase-2 rapidly translocates from the nucleus to the cytoplasm in response to apoptotic stimuli
Abstract
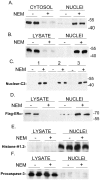
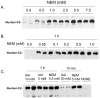
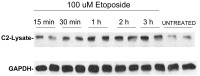
Unlike other caspases, caspase-2 appears to be a nuclear protein although immunocytochemical studies have suggested that it may also be localized to the cytosol and golgi. Where and how caspase-2 is activated in response to apoptotic signals is not clear. Earlier immunocytochemistry studies suggest that caspase-2 is activated in the nucleus and through cleavage of BID leads to increased mitochondrial permeability. More recent studies using bimolecular fluorescence complementation found that caspase-2 oligomerization that leads to activation only occurs in the cytoplasm. Thus, apoptotic signals may lead to activation of caspase-2 which may already reside in the cytoplasm or lead to release of nuclear caspase-2 to the extra-nuclear cytoplasmic compartment. It has not been possible to study release of nuclear caspase-2 to the cytoplasm by cell fractionation studies since cell lysis is known to release nuclear caspase-2 to the extra-nuclear fraction. This is similar to what is known about unliganded nuclear estrogen receptor-α (ERα ) when cells are disrupted. In this study we found that pre-treatment of cells with N-ethylmaleimide (NEM), which alkylates cysteine thiol groups in proteins, completely prevents redistribution of caspase-2 and ERα from the nucleus to the extra-nuclear fraction when cells are lysed. Using this approach we provide evidence that apoptotic signals rapidly leads to a shift of caspase-2 from the nucleus to the extra-nuclear fraction, which precedes the detection of apoptosis. These findings are consistent with a model where apoptotic signals lead to a rapid shift of caspase-2 from the nucleus to the cytoplasm where activation occurs.
Conflict of interest statement
Figures



References
-
- Hengartner MO (2000) The biochemistry of apoptosis. Nature 407: 770–776. - PubMed
-
- Lahm A, Paradisi A, Green DR, Melino G (2003) Death fold domain interaction in apoptosis. Cell Death Differ 10: 10–12. - PubMed
-
- Taylor RC, Cullen SP, Martin SJ (2008) Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 9: 231–241. - PubMed
-
- Cande C, Cecconi F, Dessen P, Kroemer G (2002) Apoptosis-inducing factor (AIF): key to the conserved caspase-independent pathways of cell death? J Cell Sci 115: 4727–4734. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

