Expression of the Pluripotency Transcription Factor OCT4 in the Normal and Aberrant Mammary Gland
- PMID: 23596564
- PMCID: PMC3622876
- DOI: 10.3389/fonc.2013.00079
Expression of the Pluripotency Transcription Factor OCT4 in the Normal and Aberrant Mammary Gland
Abstract
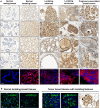
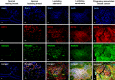
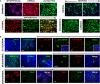
Breast cancers with lactating features, some of which are associated with pregnancy and lactation, are often poorly differentiated, lack estrogen receptor, progesterone receptor, and HER2 expression and have high mortality. Very little is known about the molecular mechanisms that drive uncontrolled cell proliferation in these tumors and confer lactating features. We have recently reported expression of OCT4 and associated embryonic stem cell self-renewal genes in the normal lactating breast and breastmilk stem cells (hBSCs). This prompted us to examine OCT4 expression in breast cancers with lactating features and compare it with that observed during normal lactation, using rare specimens of human lactating breast. In accordance with previous literature, the normal resting breast (from non-pregnant, non-lactating women) showed minimal OCT4 nuclear expression (0.9%). However, this increased in the normal lactating breast (11.4%), with further increase in lactating adenomas, lactating carcinomas, and pregnancy-associated breast cancer (30.7-48.3%). OCT4 was expressed in the epithelium and at lower levels in the stroma, and was co-localized with NANOG. Comparison of normal non-tumorigenic hBSCs with OCT4-overexpressing tumorigenic breast cell lines (OTBCs) demonstrated upregulation of OCT4, SOX2, and NANOG in both systems, but OTBCs expressed OCT4 at significantly higher levels than SOX2 and NANOG. Similar to hBSCs, OTBCs displayed multi-lineage differentiation potential, including the ability to differentiate into functional lactocytes synthesizing milk proteins both in vitro and in vivo. Based on these findings, we propose a hypothesis of normal and malignant transformation in the breast, which centers on OCT4 and its associated gene network. Although minimal expression of these embryonic genes can be seen in the breast in its resting state throughout life, a controlled program of upregulation of this gene network may be a potential regulator of the normal remodeling of the breast toward a milk-secretory organ during pregnancy and lactation. Deregulation of this gene network either within or outside pregnancy and lactation may lead to aberrant breast cell proliferation and malignant transformation, suggesting a role of these genes in both normal lactation and breast oncogenesis.
Keywords: OCT4; adult stem cell; breast cancer; breastmilk; cancer stem cell; mammary gland; self-renewal; transcription factors.
Figures


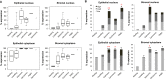
 ≤ 0.1; 0.01 < * ≤ 0.05; and 0.001 < ** ≤ 0.01.
≤ 0.1; 0.01 < * ≤ 0.05; and 0.001 < ** ≤ 0.01.

Similar articles
-
Breastmilk is a novel source of stem cells with multilineage differentiation potential.Stem Cells. 2012 Oct;30(10):2164-74. doi: 10.1002/stem.1188. Stem Cells. 2012. PMID: 22865647 Free PMC article.
-
Generation of tumor-initiating cells by exogenous delivery of OCT4 transcription factor.Breast Cancer Res. 2011 Sep 27;13(5):R94. doi: 10.1186/bcr3019. Breast Cancer Res. 2011. PMID: 21952072 Free PMC article.
-
Autochthonous mouse melanoma and mammary tumors do not express the pluripotency genes Oct4 and Nanog.PLoS One. 2013;8(2):e57465. doi: 10.1371/journal.pone.0057465. Epub 2013 Feb 28. PLoS One. 2013. PMID: 23468991 Free PMC article.
-
Pluripotency Genes and Their Functions in the Normal and Aberrant Breast and Brain.Int J Mol Sci. 2015 Nov 13;16(11):27288-301. doi: 10.3390/ijms161126024. Int J Mol Sci. 2015. PMID: 26580604 Free PMC article. Review.
-
Role of Oct4 in maintaining and regaining stem cell pluripotency.Stem Cell Res Ther. 2010 Dec 14;1(5):39. doi: 10.1186/scrt39. Stem Cell Res Ther. 2010. PMID: 21156086 Free PMC article. Review.
Cited by
-
Construction and analysis of tissue microarrays in the era of digital pathology: a pilot study targeting CDX1 and CDX2 in a colon cancer cohort of 612 patients.J Pathol Clin Res. 2017 Jan 13;3(1):58-70. doi: 10.1002/cjp2.62. eCollection 2017 Jan. J Pathol Clin Res. 2017. PMID: 28138402 Free PMC article.
-
Immune cell-mediated protection of the mammary gland and the infant during breastfeeding.Adv Nutr. 2015 May 15;6(3):267-75. doi: 10.3945/an.114.007377. Print 2015 May. Adv Nutr. 2015. PMID: 25979492 Free PMC article. Review.
-
The network of pluripotency, epithelial-mesenchymal transition, and prognosis of breast cancer.Breast Cancer (Dove Med Press). 2015 Sep 7;7:303-19. doi: 10.2147/BCTT.S71163. eCollection 2015. Breast Cancer (Dove Med Press). 2015. PMID: 26379447 Free PMC article. Review.
-
MicroRNAs in Breastmilk and the Lactating Breast: Potential Immunoprotectors and Developmental Regulators for the Infant and the Mother.Int J Environ Res Public Health. 2015 Oct 30;12(11):13981-4020. doi: 10.3390/ijerph121113981. Int J Environ Res Public Health. 2015. PMID: 26529003 Free PMC article.
-
At the dawn of a new discovery: the potential of breast milk stem cells.Adv Nutr. 2014 Nov 14;5(6):770-8. doi: 10.3945/an.114.006924. Print 2014 Nov. Adv Nutr. 2014. PMID: 25398739 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

