LGR5 is Expressed by Ewing Sarcoma and Potentiates Wnt/β-Catenin Signaling
- PMID: 23596566
- PMCID: PMC3625903
- DOI: 10.3389/fonc.2013.00081
LGR5 is Expressed by Ewing Sarcoma and Potentiates Wnt/β-Catenin Signaling
Abstract
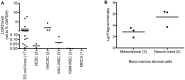
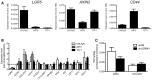
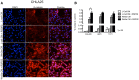
Ewing sarcoma (ES) is an aggressive bone and soft tissue tumor of putative stem cell origin that predominantly occurs in children and young adults. Although most patients with localized ES can be cured with intensive therapy, the clinical course is variable and up to one third of patients relapse following initial remission. Unfortunately, little is yet known about the biologic features that distinguish low-risk from high-risk disease or the mechanisms of ES disease progression. Recent reports have suggested that putative cancer stem cells exist in ES and may contribute to an aggressive phenotype. The cell surface receptor leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5) is a somatic stem cell marker that functions as an oncogene in several human cancers, most notably colorectal carcinoma. LGR5 is a receptor for the R-spondin (RSPO) family of ligands and RSPO-mediated activation of LGR5 potentiates Wnt/β-catenin signaling, contributing to stem cell proliferation and self-renewal. Given its presumed stem cell origin, we investigated whether LGR5 contributes to ES pathogenesis. We found that LGR5 is expressed by ES and that its expression is relatively increased in cells and tumors that display a more aggressive phenotype. In particular, LGR5 expression was increased in putative cancer stem cells. We also found that neural crest-derived stem cells express LGR5, raising the possibility that expression of LGR5 may be a feature of ES cells of origin. LGR5-high ES cells showed nuclear localization of β-catenin and robust activation of TCF reporter activity when exposed to Wnt ligand and this was potentiated by RSPO. However, modulation of LGR5 or exposure to RSPO had no impact on proliferation confirming that Wnt/β-catenin signaling in ES cells does not recapitulate signaling in epithelial cells. Together these studies show that the RSPO-LGR5-Wnt-β-catenin axis is present and active in ES and may contribute to tumor pathogenesis.
Keywords: Ewing sarcoma; LGR5; R-spondin; Wnt; stem cell; β-catenin.
Figures
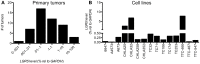


Similar articles
-
Non-equivalence of Wnt and R-spondin ligands during Lgr5+ intestinal stem-cell self-renewal.Nature. 2017 May 11;545(7653):238-242. doi: 10.1038/nature22313. Epub 2017 May 3. Nature. 2017. PMID: 28467820 Free PMC article.
-
R-Spondin potentiates Wnt/β-catenin signaling through orphan receptors LGR4 and LGR5.PLoS One. 2012;7(7):e40976. doi: 10.1371/journal.pone.0040976. Epub 2012 Jul 16. PLoS One. 2012. PMID: 22815884 Free PMC article.
-
LGR5 is associated with tumor aggressiveness in papillary thyroid cancer.Oncotarget. 2015 Oct 27;6(33):34549-60. doi: 10.18632/oncotarget.5330. Oncotarget. 2015. PMID: 26416247 Free PMC article.
-
Structure and function of LGR5: an enigmatic G-protein coupled receptor marking stem cells.Protein Sci. 2014 May;23(5):551-65. doi: 10.1002/pro.2446. Epub 2014 Mar 19. Protein Sci. 2014. PMID: 24677446 Free PMC article. Review.
-
Wnt, RSPO and Hippo Signalling in the Intestine and Intestinal Stem Cells.Genes (Basel). 2018 Jan 8;9(1):20. doi: 10.3390/genes9010020. Genes (Basel). 2018. PMID: 29316729 Free PMC article. Review.
Cited by
-
Wnt/β-catenin-activated Ewing sarcoma cells promote the angiogenic switch.JCI Insight. 2020 Jul 9;5(13):e135188. doi: 10.1172/jci.insight.135188. JCI Insight. 2020. PMID: 32544094 Free PMC article.
-
LGR5 promotes the proliferation of colorectal cancer cells via the Wnt/β-catenin signaling pathway.Oncol Lett. 2015 Jun;9(6):2859-2863. doi: 10.3892/ol.2015.3144. Epub 2015 Apr 23. Oncol Lett. 2015. PMID: 26137160 Free PMC article.
-
LGR5 and Downstream Intracellular Signaling Proteins Play Critical Roles in the Cell Proliferation of Neuroblastoma, Meningioma and Pituitary Adenoma.Exp Neurobiol. 2019 Oct 31;28(5):628-641. doi: 10.5607/en.2019.28.5.628. Exp Neurobiol. 2019. PMID: 31698554 Free PMC article.
-
New Insights about the Wnt/β-Catenin Signaling Pathway in Primary Bone Tumors and Their Microenvironment: A Promising Target to Develop Therapeutic Strategies?Int J Mol Sci. 2019 Jul 31;20(15):3751. doi: 10.3390/ijms20153751. Int J Mol Sci. 2019. PMID: 31370265 Free PMC article. Review.
-
LGR5 and BMI1 Increase Pig Intestinal Epithelial Cell Proliferation by Stimulating WNT/β-Catenin Signaling.Int J Mol Sci. 2018 Mar 30;19(4):1036. doi: 10.3390/ijms19041036. Int J Mol Sci. 2018. PMID: 29601474 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

