Principles underlying rational design of live attenuated influenza vaccines
- PMID: 23596576
- PMCID: PMC3623510
- DOI: 10.7774/cevr.2012.1.1.35
Principles underlying rational design of live attenuated influenza vaccines
Abstract
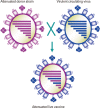
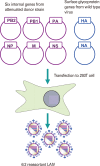
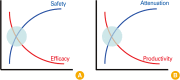
Despite recent innovative advances in molecular virology and the developments of vaccines, influenza virus remains a serious burden for human health. Vaccination has been considered a primary countermeasure for prevention of influenza infection. Live attenuated influenza vaccines (LAIVs) are particularly attracting attention as an effective strategy due to several advantages over inactivated vaccines. Cold-adaptation, as a classical means for attenuating viral virulence, has been successfully used for generating safe and effective donor strains of LAIVs against seasonal epidemics and occasional pandemics. Recently, the advent of reverse genetics technique expedited a variety of rational strategies to broaden the pool of LAIVs. Considering the breadth of antigenic diversity of influenza virus, the pool of LAIVs is likely to equip us with better options for controlling influenza pandemics. With a brief reflection on classical attenuating strategies used at the initial stage of development of LAIVs, especially on the principles underlying the development of cold-adapted LAIVs, we further discuss and outline other attenuation strategies especially with respect to the rationales for attenuation, and their practicality for mass production. Finally, we propose important considerations for a rational vaccine design, which will provide us with practical guidelines for improving the safety and effectiveness of LAIVs.
Keywords: Attenuation strategy; Cold-adaptation; Cross protection; Influenza live attenuated vaccine.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
Similar articles
-
Next generation live-attenuated influenza vaccine platforms.Expert Rev Vaccines. 2022 Aug;21(8):1097-1110. doi: 10.1080/14760584.2022.2072301. Epub 2022 May 5. Expert Rev Vaccines. 2022. PMID: 35502639 Review.
-
Immune Responses Elicited by Live Attenuated Influenza Vaccines as Correlates of Universal Protection against Influenza Viruses.Vaccines (Basel). 2021 Apr 7;9(4):353. doi: 10.3390/vaccines9040353. Vaccines (Basel). 2021. PMID: 33916924 Free PMC article. Review.
-
Alternative Strategy for a Quadrivalent Live Attenuated Influenza Virus Vaccine.J Virol. 2018 Oct 12;92(21):e01025-18. doi: 10.1128/JVI.01025-18. Print 2018 Nov 1. J Virol. 2018. PMID: 30135124 Free PMC article.
-
Comparative Study of the Temperature Sensitive, Cold Adapted and Attenuated Mutations Present in the Master Donor Viruses of the Two Commercial Human Live Attenuated Influenza Vaccines.Viruses. 2019 Oct 10;11(10):928. doi: 10.3390/v11100928. Viruses. 2019. PMID: 31658679 Free PMC article.
-
Generation of DelNS1 Influenza Viruses: a Strategy for Optimizing Live Attenuated Influenza Vaccines.mBio. 2019 Sep 17;10(5):e02180-19. doi: 10.1128/mBio.02180-19. mBio. 2019. PMID: 31530680 Free PMC article.
Cited by
-
Applications of synthetic biology in medical and pharmaceutical fields.Signal Transduct Target Ther. 2023 May 11;8(1):199. doi: 10.1038/s41392-023-01440-5. Signal Transduct Target Ther. 2023. PMID: 37169742 Free PMC article. Review.
-
Host defense mechanism-based rational design of live vaccine.PLoS One. 2013 Oct 2;8(10):e75043. doi: 10.1371/journal.pone.0075043. eCollection 2013. PLoS One. 2013. PMID: 24098364 Free PMC article.
-
RanDeL-Seq: a High-Throughput Method to Map Viral cis- and trans-Acting Elements.mBio. 2021 Jan 19;12(1):e01724-20. doi: 10.1128/mBio.01724-20. mBio. 2021. PMID: 33468683 Free PMC article.
-
Development of vaccines for SARS-CoV-2.F1000Res. 2020 Aug 17;9:F1000 Faculty Rev-991. doi: 10.12688/f1000research.25998.1. eCollection 2020. F1000Res. 2020. PMID: 32850116 Free PMC article. Review.
-
Genetic-Code-Expansion Strategies for Vaccine Development.Chembiochem. 2020 Dec 1;21(23):3291-3300. doi: 10.1002/cbic.202000343. Epub 2020 Jul 30. Chembiochem. 2020. PMID: 32608153 Free PMC article. Review.
References
-
- Lamb RA, Krug RM. Orthomyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, Griffin DE, et al., editors. Fields virology. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1487–1532.
LinkOut - more resources
Full Text Sources
Other Literature Sources

