Recombinant influenza viruses as delivery vectors for hepatis B virus epitopes
- PMID: 23596580
- PMCID: PMC3623514
- DOI: 10.7774/cevr.2012.1.1.77
Recombinant influenza viruses as delivery vectors for hepatis B virus epitopes
Abstract
Purpose: Neuraminidase (NA) of influenza virus contains stalk region that shows a great deal of variability in both amino acid sequence and length. In this paper, we investigated generation of recombinant influenza viruses that had hepatitis B virus (HBV) B cell epitopes in the NA stalk region as a dual vaccine candidate.
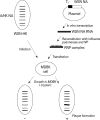
Materials and methods: We used the WSH-HK reassortant helper virus for rescue of recombinant influenza virus containing HBV epitopes and reverse genetic protocol based on the use of micrococcal nuclease-treated virus cores for reconstitution of ribonucleoproteins.
Results: We successfully generated a chimeric influenza viruses which contained 22 amino acid peptides in the stalk region derived from the surface and pre-surface protein HBV. The growth kinetics of the recombinant viruses was investigated after infection of Madin-Darby canine kidney (MDCK) and Madin-Darby bovine kidney (MDBK) cells and the rIV-BVPreS virus showed higher titer than other viruses in MDCK cells. We also confirmed the presence of HBV epitopes in the chimeric viruses by enzyme-linked immunosorbent assay (ELISA) using anti-HBV polyclonal antibody. When the ratio of recombinant virus verse wild type virus was calculated by ELISA, recombinant viruses exhibited 2 fold higher values than the wild type virus.
Conclusion: These results suggest that chimeric influenza virus which contained foreign antigens can be used as dual vaccine against both HBV and influenza viruses.
Keywords: Chimeric virus; Dual vaccine; NA stalk.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

Similar articles
-
Neuraminidase inhibitor susceptibility and neuraminidase enzyme kinetics of human influenza A and B viruses circulating in Thailand in 2010-2015.PLoS One. 2018 Jan 11;13(1):e0190877. doi: 10.1371/journal.pone.0190877. eCollection 2018. PLoS One. 2018. PMID: 29324781 Free PMC article.
-
Influenza Neuraminidase Subtype N1: Immunobiological Properties and Functional Assays for Specific Antibody Response.PLoS One. 2016 Apr 7;11(4):e0153183. doi: 10.1371/journal.pone.0153183. eCollection 2016. PLoS One. 2016. PMID: 27054879 Free PMC article.
-
Extending the Stalk Enhances Immunogenicity of the Influenza Virus Neuraminidase.J Virol. 2019 Aug 28;93(18):e00840-19. doi: 10.1128/JVI.00840-19. Print 2019 Sep 15. J Virol. 2019. PMID: 31375573 Free PMC article.
-
Increase in viral yield in eggs and MDCK cells of reassortant H5N1 vaccine candidate viruses caused by insertion of 38 amino acids into the NA stalk.Vaccine. 2011 Oct 19;29(45):8032-41. doi: 10.1016/j.vaccine.2011.08.054. Epub 2011 Aug 22. Vaccine. 2011. PMID: 21864614
-
Systematic evaluation of suspension MDCK cells, adherent MDCK cells, and LLC-MK2 cells for preparing influenza vaccine seed virus.Vaccine. 2019 Oct 8;37(43):6526-6534. doi: 10.1016/j.vaccine.2019.08.064. Epub 2019 Sep 6. Vaccine. 2019. PMID: 31500967
Cited by
-
Induced immunity against hepatitis B virus.World J Hepatol. 2015 Jun 28;7(12):1660-70. doi: 10.4254/wjh.v7.i12.1660. World J Hepatol. 2015. PMID: 26140085 Free PMC article. Review.
References
-
- Baum C, Schambach A, Bohne J, Galla M. Retrovirus vectors: toward the plentivirus? Mol Ther. 2006;13:1050–1063. - PubMed
-
- Young LS, Searle PF, Onion D, Mautner V. Viral gene therapy strategies: from basic science to clinical application. J Pathol. 2006;208:299–318. - PubMed
-
- Arribillaga L, de Cerio AL, Sarobe P, et al. Vaccination with an adenoviral vector encoding hepatitis C virus (HCV) NS3 protein protects against infection with HCV-recombinant vaccinia virus. Vaccine. 2002;21:202–210. - PubMed
-
- Fournillier A, Gerossier E, Evlashev A, et al. An accelerated vaccine schedule with a poly-antigenic hepatitis C virus MVA-based candidate vaccine induces potent, long lasting and in vivo cross-reactive T cell responses. Vaccine. 2007;25:7339–7353. - PubMed
LinkOut - more resources
Full Text Sources

