Current status of vaccine development for tularemia preparedness
- PMID: 23596588
- PMCID: PMC3623498
- DOI: 10.7774/cevr.2013.2.1.34
Current status of vaccine development for tularemia preparedness
Abstract
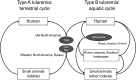
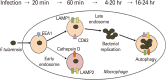
Tularemia is a high-risk infectious disease caused by Gram-negative bacterium Francisella tularensis. Due to its high fatality at very low colony-forming units (less than 10), F. tularensis is considered as a powerful potential bioterrorism agent. Vaccine could be the most efficient way to prevent the citizen from infection of F. tularensis when the bioterrorism happens, but officially approved vaccine with both efficacy and safety is not developed yet. Research for the development of tularemia vaccine has been focusing on the live attenuated vaccine strain (LVS) for long history, still there are no LVS confirmed for the safety which should be an essential factor for general vaccination program. Furthermore the LVS did not show protection efficacy against high-risk subspecies tularensis (type A) as high as the level against subspecies holarctica (type B) in human. Though the subunit or recombinant vaccine candidates have been considered for better safety, any results did not show better prevention efficacy than the LVS candidate against F. tularensis infection. Currently there are some more trials to develop vaccine using mutant strains or nonpathogenic F. novicida strain, but it did not reveal effective candidates overwhelming the LVS either. Difference in the protection efficacy of LVS against type A strain in human and the low level protection of many subunit or recombinant vaccine candidates lead the scientists to consider the live vaccine development using type A strain could be ultimate answer for the tularemia vaccine development.
Keywords: Bioterrorism agent; Fracisella tularensis; Preparedness; Tularemia; Vaccines.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Saslaw S, Eigelsbach HT, Prior JA, Wilson HE, Carhart S. Tularemia vaccine study. II. Respiratory challenge. Arch Intern Med. 1961;107:702–714. - PubMed
-
- Saslaw S, Eigelsbach HT, Wilson HE, Prior JA, Carhart S. Tularemia vaccine study. I. Intracutaneous challenge. Arch Intern Med. 1961;107:689–701. - PubMed
-
- Dennis DT, Inglesby TV, Henderson DA, et al. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–2773. - PubMed
-
- Keim P, Johansson A, Wagner DM. Molecular epidemiology, evolution, and ecology of Francisella. Ann N Y Acad Sci. 2007;1105:30–66. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

