Therapeutic targeting of CPT-11 induced diarrhea: a case for prophylaxis
- PMID: 23597015
- PMCID: PMC4004171
- DOI: 10.2174/1389450111314070007
Therapeutic targeting of CPT-11 induced diarrhea: a case for prophylaxis
Abstract
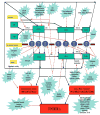
CPT-11 (irinotecan), a DNA topoisomerase I inhibitor is one of the main treatments for colorectal cancer. The main dose limiting toxicities are neutropenia and late onset diarrhea. Though neutropenia is manageable, CPT-11 induced diarrhea is frequently severe, resulting in hospitalizations, dose reductions or omissions leading to ineffective treatment administration. Many potential agents have been tested in preclinical and clinical studies to prevent or ameliorate CPT-11 induced late onset diarrhea. It is predicted that prophylaxis of CPT-11 induced diarrhea will reduce sub-therapeutic dosing as well as hospitalizations and will eventually lead to dose escalations resulting in better response rates. This article reviews various experimental agents and strategies employed to prevent this debilitating toxicity. Covered topics include schedule/dose modification, intestinal alkalization, structural/chemical modification, genetic testing, anti-diarrheal therapies, transporter (ABCB1, ABCC2, BCRP2) inhibitors, enzyme (β-glucuronidase, UGT1A1, CYP3A4, carboxylesterase, COX-2) inducers and inhibitors, probiotics, antibiotics, adsorbing agents, cytokine and growth factor activators and inhibitors and other miscellaneous agents.
Conflict of interest statement
The author(s) confirm that this article content has no conflicts of interest.
Figures
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. - PubMed
-
- Tanizawa A, Fujimori A, Fujimori Y, Pommier Y. Comparison of topoisomerase I inhibition, DNA damage, and cytotoxicity of camptothecin derivatives presently in clinical trials. J Natl Cancer Inst. 1994;86(11):836–42. - PubMed
-
- Smith NF, Figg WD, Sparreboom A. Pharmacogenetics of irinotecan metabolism and transport: an update. Toxicol In vitro. 2006;20(2):163–75. - PubMed
-
- NCCN. [accessed Nov 16, 2012];National Comprehensive Cancer Network Guidelines. http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
-
- Rosen LS. Irinotecan in lymphoma, leukemia, and breast, pancreatic, ovarian, and small-cell lung cancers. Oncology (Williston Park) 1998;12(8 Suppl 6):103–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials