Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials
- PMID: 23597181
- PMCID: PMC3648456
- DOI: 10.1186/1741-7015-11-108
Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials
Abstract
Background: Testosterone therapy is increasingly promoted. No randomized placebo-controlled trial has been implemented to assess the effect of testosterone therapy on cardiovascular events, although very high levels of androgens are thought to promote cardiovascular disease.
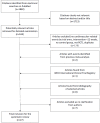
Methods: A systematic review and meta-analysis was conducted of placebo-controlled randomized trials of testosterone therapy among men lasting 12+ weeks reporting cardiovascular-related events. We searched PubMed through the end of 2012 using "("testosterone" or "androgen") and trial and ("random*")" with the selection limited to studies of men in English, supplemented by a bibliographic search of the World Health Organization trial registry. Two reviewers independently searched, selected and assessed study quality with differences resolved by consensus. Two statisticians independently abstracted and analyzed data, using random or fixed effects models, as appropriate, with inverse variance weighting.
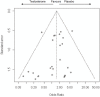
Results: Of 1,882 studies identified 27 trials were eligible including 2,994, mainly older, men who experienced 180 cardiovascular-related events. Testosterone therapy increased the risk of a cardiovascular-related event (odds ratio (OR) 1.54, 95% confidence interval (CI) 1.09 to 2.18). The effect of testosterone therapy varied with source of funding (P-value for interaction 0.03), but not with baseline testosterone level (P-value for interaction 0.70). In trials not funded by the pharmaceutical industry the risk of a cardiovascular-related event on testosterone therapy was greater (OR 2.06, 95% CI 1.34 to 3.17) than in pharmaceutical industry funded trials (OR 0.89, 95% CI 0.50 to 1.60).
Conclusions: The effects of testosterone on cardiovascular-related events varied with source of funding. Nevertheless, overall and particularly in trials not funded by the pharmaceutical industry, exogenous testosterone increased the risk of cardiovascular-related events, with corresponding implications for the use of testosterone therapy.
Figures


Comment in
-
Need for standardising adverse event reporting in testosterone trials.Evid Based Med. 2014 Feb;19(1):32-3. doi: 10.1136/eb-2013-101402. Epub 2013 Jul 17. Evid Based Med. 2014. PMID: 23863945 No abstract available.
References
-
- Sartorius G, Spasevska S, Idan A, Turner L, Forbes E, Zamojska A, Allan CA, Ly LP, Conway AJ, McLachlan RI, Handelsman DJ. Serum testosterone, dihydrotestosterone and estradiol concentrations in older men self-reporting very good health: the healthy man study. Clin Endocrinol (Oxf) 2012;77:755–763. doi: 10.1111/j.1365-2265.2012.04432.x. - DOI - PubMed
-
- Levine GN, D’Amico AV, Berger P, Clark PE, Eckel RH, Keating NL, Milani RV, Sagalowsky AI, Smith MR, Zakai N. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. CA Cancer J Clin. 2010;60:194–201. doi: 10.3322/caac.20061. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

