Intestinal alkaline phosphatase prevents antibiotic-induced susceptibility to enteric pathogens
- PMID: 23598380
- PMCID: PMC3855644
- DOI: 10.1097/SLA.0b013e31828fae14
Intestinal alkaline phosphatase prevents antibiotic-induced susceptibility to enteric pathogens
Abstract
Objective: To determine the efficacy of oral supplementation of the gut enzyme intestinal alkaline phosphatase (IAP) in preventing antibiotic-associated infections from Salmonella enterica serovar Typhimurium (S. Typhimurium) and Clostridium difficile.
Background: The intestinal microbiota plays a pivotal role in human health and well-being. Antibiotics inherently cause dysbiosis, an imbalance in the number and composition of intestinal commensal bacteria, which leads to susceptibility to opportunistic bacterial infections. Previously, we have shown that IAP preserves the normal homeostasis of intestinal microbiota and that oral supplementation with calf IAP (cIAP) rapidly restores the normal gut flora. We hypothesized that oral IAP supplementation would protect against antibiotic-associated bacterial infections.
Methods: C57BL/6 mice were treated with antibiotic(s) ± cIAP in the drinking water, followed by oral gavage of S. Typhimurium or C. difficile. Mice were observed for clinical conditions and mortality. After a defined period of time, mice were killed and investigated for hematological, inflammatory, and histological changes.
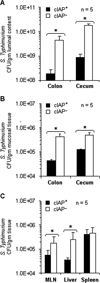
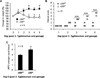
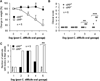
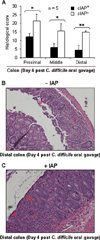
Results: We observed that oral supplementation with cIAP during antibiotic treatment protects mice from infections with S. Typhimurium as well as with C. difficile. Animals given IAP maintained their weight, had reduced clinical severity and gut inflammation, and showed improved survival.
Conclusions: Oral IAP supplementation protected mice from antibiotic-associated bacterial infections. We postulate that oral IAP supplementation could represent a novel therapy to protect against antibiotic-associated diarrhea (AAD), C. difficile-associated disease (CDAD), and other enteric infections in humans.
Conflict of interest statement
Figures


References
-
- Miller CP, Bohnhoff M. Changes in the Mouse's Enteric Microflora Associated with Enhanced Susceptibility to Salmonella Infection Following Streptomycin Treatment. J Infect Dis. 1963;113:59–66. - PubMed
-
- Chen X, Katchar K, Goldsmith JD, et al. A mouse model of Clostridium difficile-associated disease. Gastroenterology. 2008;135(6):1984–1992. - PubMed
-
- Glynn MK, Reddy V, Hutwagner L, et al. Prior antimicrobial agent use increases the risk of sporadic infections with multidrug-resistant Salmonella enterica serotype Typhimurium: a FoodNet case-control study, 1996–1997. Clin Infect Dis. 2004;38(Suppl 3):S227–S236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

