Human umbilical cord blood-derived mesenchymal stem cells in the cultured rabbit intervertebral disc: a novel cell source for disc repair
- PMID: 23598901
- PMCID: PMC4238943
- DOI: 10.1097/PHM.0b013e31825f148a
Human umbilical cord blood-derived mesenchymal stem cells in the cultured rabbit intervertebral disc: a novel cell source for disc repair
Abstract
Objective: Back pain associated with symptomatic disc degeneration is a common clinical condition. Intervertebral disc (IVD) cell apoptosis and senescence increase with aging and degeneration. Repopulating the IVD with cells that could produce and maintain extracellular matrix would be an alternative therapy to surgery. The objective of this study was to determine the potential of human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) as a novel cell source for disc repair. In this study, we intended to confirm the potential for hUCB-MSCs to differentiate and display a chondrocyte-like phenotype after culturing in micromass and after injection into the rabbit IVD explant culture. We also wanted to confirm hUCB-MSC survival after transplantation into the IVD explant culture.
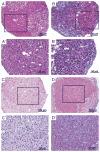
Design: This study consisted of micromass cultures and in vitro rabbit IVD explant cultures to assess hUCB-MSC survival and differentiation to display chondrocyte-like phenotype. First, hUCB-MSCs were cultured in micromass and stained with Alcian blue dye. Second, to confirm cell survival, hUCB-MSCs were labeled with an infrared dye and a fluorescent dye before injection into whole rabbit IVD explants (host). IVD explants were then cultured for 4 wks. Cell survival was confirmed by two independent techniques: an imaging system detecting the infrared dye at the organ level and fluorescence microscopy detecting fluorescent dye at the cellular level. Cell viability was assessed by staining the explant with CellTracker green, a membrane-permeant tracer specific for live cells. Human type II collagen gene expression (from the graft) was assessed by polymerase chain reaction.
Results: We have shown that hUCB-MSCs cultured in micromass are stained blue with Alcian blue dye, which suggests that proteoglycan-rich extracellular matrix is produced. In the cultured rabbit IVD explants, hUCB-MSCs survived for at least 4 wks and expressed the human type II collagen gene, suggesting that the injected hUCB-MSCs are differentiating into a chondrocyte-like lineage.
Conclusions: This study demonstrates the abiity of hUBC-MSCs to survive and assume a chondrocyte-like phenotype when injected into the rabbit IVD. These data support the potential for hUBC-MSCs as a cell source for disc repair. Further measures of the host response to the injection and studies in animal models are needed before trials in humans.
Figures




References
-
- United States Bone and Joint Decade. The Burden of Musculoskeletal Diseases in the United States. Rosemont, IL: The American Academy of Orthopaedic Surgeons; 2008.
-
- Anderson DG, Tannoury C. Molecular pathogenic factors in symptomatic disc degeneration. Spine J. 2005;5:260S–6S. - PubMed
-
- Gruber HE, Hanley EN., Jr Analysis of aging and degeneration of the human intervertebral disc. Comparison of surgical specimens with normal controls. Spine. 1998;23:751–7. - PubMed
-
- Zhang Y, An HS, Tannoury C, et al. Biological treatment for degenerative disc disease: Implications for the field of physical medicine and rehabilitation. Am J Phys Med Rehabil. 2008;87:694–702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

