Topical interleukin 1 receptor antagonist for treatment of dry eye disease: a randomized clinical trial
- PMID: 23599118
- PMCID: PMC4167802
- DOI: 10.1001/jamaophthalmol.2013.195
Topical interleukin 1 receptor antagonist for treatment of dry eye disease: a randomized clinical trial
Abstract
Importance: The immunopathogenic mechanisms of dry eye disease (DED), one of the most common ophthalmic conditions, is incompletely understood. Data from this prospective, double-masked, randomized trial demonstrate that targeting interleukin 1 (IL-1) by topical application of an IL-1 antagonist is efficacious in significantly reducing DED-related patient symptoms and corneal epitheliopathy.
Objective: To evaluate the safety and efficacy of treatment with the topical IL-1 receptor antagonist anakinra (Kineret; Amgen Inc) in patients having DED associated with meibomian gland dysfunction.
Design and setting: Prospective phase 1/2, randomized, double-masked, vehicle-controlled clinical trial.
Participants: Seventy-five patients with refractory DED.
Interventions: Participants were randomized to receive treatment with topical anakinra, 2.5% (n = 30), anakinra, 5% (n = 15), or vehicle (1% carboxymethylcellulose) (n = 30) 3 times daily for 12 weeks.
Main outcomes and measures: Primary outcomes were corneal fluorescein staining (CFS), complete bilateral CFS clearance, dry eye-related symptoms as measured by the Ocular Surface Disease Index, tear film breakup time, and meibomian gland secretion quality.
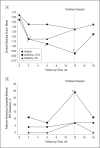
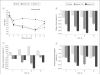
Results: Topical anakinra was well tolerated compared with vehicle, with no reports of serious adverse reactions attributable to the therapy. After 12 weeks of therapy, participants treated with anakinra, 2.5%, achieved a 46% reduction in their mean CFS score (P = .12 compared with vehicle and P < .001 compared with baseline); participants treated with anakinra, 5%, achieved a 17% reduction in their mean CFS score (P = .88 compared with vehicle and P = .33 compared with baseline); and patients treated with vehicle achieved a 19% reduction in their mean CFS score (P = .11). Complete bilateral CFS clearance was noted in 8 of 28 patients (29%) treated with anakinra, 2.5%, vs in 2 of 29 patients (7%) treated with vehicle (P = .03). By week 12, treatment with anakinra, 2.5%, and treatment with anakinra, 5%, led to significant reductions in symptoms of 30% and 35%, respectively (P = .02 and P = .01, respectively, compared with vehicle); treatment with vehicle led to a 5% reduction in symptoms.
Conclusions and relevance: Treatment with topical anakinra, 2.5%, for 12 weeks was safe and significantly reduced symptoms and corneal epitheliopathy in patients with DED. These data suggest that the use of an IL-1 antagonist may have a role as a novel therapeutic option for patients with DED. TRIAL REGISTRATION clinicaltrials.gov Identifier: NCT00681109.
Figures

References
-
- International Dry Eye WorkShop Study Group The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):75–92. - PubMed
-
- Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318–326. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

