10 x '20 Progress--development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America
- PMID: 23599308
- PMCID: PMC3707426
- DOI: 10.1093/cid/cit152
10 x '20 Progress--development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America
Abstract
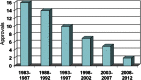
Infections caused by antibiotic-resistant bacteria, especially the "ESKAPE" pathogens, continue to increase in frequency and cause significant morbidity and mortality. New antimicrobial agents are greatly needed to treat infections caused by gram-negative bacilli (GNB) resistant to currently available agents. The Infectious Diseases Society of America (IDSA) continues to propose legislative, regulatory, and funding solutions to this continuing crisis. The current report updates the status of development and approval of systemic antibiotics in the United States as of early 2013. Only 2 new antibiotics have been approved since IDSA's 2009 pipeline status report, and the number of new antibiotics annually approved for marketing in the United States continues to decline. We identified 7 drugs in clinical development for treatment of infections caused by resistant GNB. None of these agents was included in our 2009 list of antibacterial compounds in phase 2 or later development, but unfortunately none addresses the entire spectrum of clinically relevant GNB resistance. Our survey demonstrates some progress in development of new antibacterial drugs that target infections caused by resistant GNB, but progress remains alarmingly elusive. IDSA stresses our conviction that the antibiotic pipeline problem can be solved by the collaboration of global leaders to develop creative incentives that will stimulate new antibacterial research and development. Our aim is the creation of a sustainable global antibacterial drug research and development enterprise with the power in the short term to develop 10 new, safe, and efficacious systemically administered antibiotics by 2020 as called for in IDSA's "10 × '20 Initiative."
Keywords: antibacterial agents; antibiotic pipeline; antimicrobials; clinical trials; drug development; gram-negative bacilli.
Figures
References
-
- Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis. 2008;197:1079–81. - PubMed
-
- Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. - PubMed
-
- Kallen AJ, Hidron AI, Patel J, Srinivasan A. Multidrug resistance among gram-negative pathogens that caused healthcare-associated infections reported to the National Healthcare Safety Network, 2006–2008. Infect Control Hosp Epidemiol. 2010;31:528–31. - PubMed
-
- Arias CA, Murray BE. Antibiotic-resistant bugs in the 21st century—a clinical super-challenge. N Engl J Med. 2009;360:439–43. - PubMed
-
- World Health Organization. The evolving threat of antimicrobial resistance—options for action. 2012. Available at http://whqlibdoc.who.int/publications/2012/9789241503181_eng.pdf . Accessed 30 July 2012.
Publication types
MeSH terms
Substances
Grants and funding
- HHSN267200700051C/HD/NICHD NIH HHS/United States
- K24 HD058735/HD/NICHD NIH HHS/United States
- R01 HD057956/HD/NICHD NIH HHS/United States
- R01 AI072219/AI/NIAID NIH HHS/United States
- 1R01HD057956-02/HD/NICHD NIH HHS/United States
- R01 AI063517/AI/NIAID NIH HHS/United States
- R01AI063517/AI/NIAID NIH HHS/United States
- U10 HD045962/HD/NICHD NIH HHS/United States
- 1U10-HD45962-06/HD/NICHD NIH HHS/United States
- 1K24HD058735-01/HD/NICHD NIH HHS/United States
- R01AI072219/AI/NIAID NIH HHS/United States
- R01 FD003519/FD/FDA HHS/United States
- 1R01FD003519-01/FD/FDA HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical