A novel oral nutraceutical formula of omega-3 and omega-6 fatty acids with vitamins (PLP10) in relapsing remitting multiple sclerosis: a randomised, double-blind, placebo-controlled proof-of-concept clinical trial
- PMID: 23599375
- PMCID: PMC3641495
- DOI: 10.1136/bmjopen-2012-002170
A novel oral nutraceutical formula of omega-3 and omega-6 fatty acids with vitamins (PLP10) in relapsing remitting multiple sclerosis: a randomised, double-blind, placebo-controlled proof-of-concept clinical trial
Abstract
Objective: To assess whether three novel interventions, formulated based on a systems medicine therapeutic concept, reduced disease activity in patients with relapsing-remitting multiple sclerosis (MS) who were either treated or not with disease-modifying treatment.
Design: A 30-month randomised, double-blind, placebo-controlled, parallel design, phase II proof-of-concept clinical study.
Settings: Cyprus Institute of Neurology and Genetics.
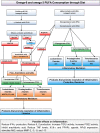
Participants: 80 participants were randomised into four groups of 20 each. A total of 41 (51%) patients completed the 30-month trial. The eligibility criteria were an age of 18-65; a diagnosis of relapsing-remitting MS according to the McDonald criteria; a score of 0.0-5.5 on the Expanded Disability Status Scale (EDSS); MRI showing lesions consistent with MS; at least one documented clinical relapse and either receiving or not a disease-modifying treatment within the 24-month period before enrolment in the study. Patients were excluded because of a recent (<30 days) relapse, prior immunosuppressant or monoclonal antibody therapy, pregnancy or nursing, other severe disease compromising organ function, progressive MS, history of recent drug or alcohol abuse, use of any additional food supplements, vitamins or any form of polyunsaturated fatty acids, and a history of severe allergic or anaphylactic reactions or known specific nutritional hypersensitivity.
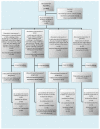
Interventions: The first intervention (A) was composed of Ω-3 and Ω-6 polyunsaturated fatty acids at 1:1 wt/wt. Specifically, the Ω-3 fatty acids were docosahexaenoic acid and eicosapentaenoic acid at 3:1 wt/wt, and the Ω-6 fatty acids were linoleic acid and γ-linolenic acid at 2:1 wt/wt. This intervention also included minor quantities of other specific polyunsaturated, monounsaturated and saturated fatty acids as well as vitamin A and vitamin E (α-tocopherol). The second intervention (B, PLP10) was a combination of A and γ-tocopherol. The third intervention (C) was γ-tocopherol alone. The fourth group of 20 participants received placebo. The interventions were administered per os (by mouth) once daily, 30 min before dinner for 30 months.
Main outcome measures: The primary end point was the annualised relapse rate (ARR) of the three interventions versus the placebo at 2 years. The secondary end point was the time to confirmed disability progression at 2 years.
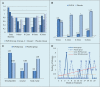
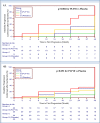
Results: A total of 41 (51%) patients completed the 30-month trial. Overall, for the per-protocol analysis of the 2-year primary end point, eight relapses were recorded in the PLP10 group (n=10; 0.40 ARR) versus 25 relapses in the placebo group (n=12; 1.04 ARR), representing a 64% adjusted relative rate reduction for the PLP10 group (RRR 0.36, 95% CI 0.15 to 0.87, p=0.024). In a subgroup analysis that excluded patients on monoclonal antibody (natalizumab) treatment, the observed adjusted RRR became stronger (72%) over the 2 years (RRR 0.28, 95% CI 0.10 to 0.79, p=0.016). The per-protocol analysis for the secondary outcome at 2 years, the time to disability progression, was significantly longer only for PLP10. The cumulative probability of disability progression at 2 years was 10% in the PLP10 group and 58% in the placebo group (unadjusted log-rank p=0.019). In a subgroup analysis that excluded patients on natalizumab, the cumulative probability of progression was 10% for the 10 patients in the PLP10 group and 70% for the 12 patients in the placebo group, representing a relative 86% decrease in the risk of the sustained progression of disability in the PLP10 group (unadjusted log-rank p=0.006; adjusted HR, 0.11; 95% CI 0.01 to 0.97, p=0.047). No adverse events were reported. Interventions A (10 patients) and C (9 patients) showed no significant efficacy.
Conclusions: In this small proof-of-concept, randomised, double-blind clinical trial; the PLP10 treatment significantly reduced the ARR and the risk of sustained disability progression without any reported serious adverse events. Larger studies are needed to further assess the safety and efficacy of PLP10.
Trial registration: International Standard Randomised Controlled Trial, number ISRCTN87818535.
Figures
References
-
- Baranzini SE, Oksenberg JR, Hauser SL. New insights into the genetics of multiple sclerosis. J Rehabil Res Dev 2002;39:201–10 - PubMed
-
- Lobo I. Multifactorial inheritance and genetic disease. Nat Educ 2008;1(1) http://www.nature.com/scitable/topicpage/multifactorial-inheritance-and-....
-
- Compston A, Coles A. Multiple sclerosis. Lancet 2002;359:1221. - PubMed
-
- Evans PH. Free radicals in brain metabolism and pathology. Br Med Bull 1993;49:577–87 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
