Migrating partial seizures of infancy: expansion of the electroclinical, radiological and pathological disease spectrum
- PMID: 23599387
- PMCID: PMC3634200
- DOI: 10.1093/brain/awt073
Migrating partial seizures of infancy: expansion of the electroclinical, radiological and pathological disease spectrum
Abstract
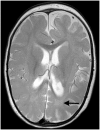
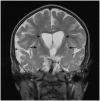
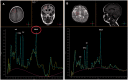
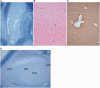
Migrating partial seizures of infancy, also known as epilepsy of infancy with migrating focal seizures, is a rare early infantile epileptic encephalopathy with poor prognosis, presenting with focal seizures in the first year of life. A national surveillance study was undertaken in conjunction with the British Paediatric Neurology Surveillance Unit to further define the clinical, pathological and molecular genetic features of this disorder. Fourteen children with migrating partial seizures of infancy were reported during the 2 year study period (estimated prevalence 0.11 per 100,000 children). The study has revealed that migrating partial seizures of infancy is associated with an expanded spectrum of clinical features (including severe gut dysmotility and a movement disorder) and electrographic features including hypsarrhythmia (associated with infantile spasms) and burst suppression. We also report novel brain imaging findings including delayed myelination with white matter hyperintensity on brain magnetic resonance imaging in one-third of the cohort, and decreased N-acetyl aspartate on magnetic resonance spectroscopy. Putaminal atrophy (on both magnetic resonance imaging and at post-mortem) was evident in one patient. Additional neuropathological findings included bilateral hippocampal gliosis and neuronal loss in two patients who had post-mortem examinations. Within this cohort, we identified two patients with mutations in the newly discovered KCNT1 gene. Comparative genomic hybridization array, SCN1A testing and genetic testing for other currently known early infantile epileptic encephalopathy genes (including PLCB1 and SLC25A22) was non-informative for the rest of the cohort.
Figures


References
-
- Caraballo RH, Fontana E, Darra F, Cassar L, Negrini F, Fiorini E, et al. Migrating focal seizures in infancy: analysis of the electro-clinical patterns in 17 patients. J Child Neurol. 2008;23:497–506. - PubMed
-
- Chien YH, Lin MI, Weng WC, Du JC, Lee WT. Dextromethorphan in the treatment of early myoclonic encephalopathy evolving into migrating partial seizures in infancy. J Formos Med Assoc. 2012;111:290–4. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

