Relationship between tendon stiffness and failure: a metaanalysis
- PMID: 23599401
- PMCID: PMC3727010
- DOI: 10.1152/japplphysiol.01449.2012
Relationship between tendon stiffness and failure: a metaanalysis
Abstract
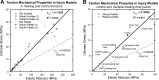
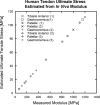
Tendon is a highly specialized, hierarchical tissue designed to transfer forces from muscle to bone; complex viscoelastic and anisotropic behaviors have been extensively characterized for specific subsets of tendons. Reported mechanical data consistently show a pseudoelastic, stress-vs.-strain behavior with a linear slope after an initial toe region. Many studies report a linear, elastic modulus, or Young's modulus (hereafter called elastic modulus) and ultimate stress for their tendon specimens. Individually, these studies are unable to provide a broader, interstudy understanding of tendon mechanical behavior. Herein we present a metaanalysis of pooled mechanical data from a representative sample of tendons from different species. These data include healthy tendons and those altered by injury and healing, genetic modification, allograft preparation, mechanical environment, and age. Fifty studies were selected and analyzed. Despite a wide range of mechanical properties between and within species, elastic modulus and ultimate stress are highly correlated (R(2) = 0.785), suggesting that tendon failure is highly strain-dependent. Furthermore, this relationship was observed to be predictable over controlled ranges of elastic moduli, as would be typical of any individual species. With the knowledge gained through this metaanalysis, noninvasive tools could measure elastic modulus in vivo and reasonably predict ultimate stress (or structural compromise) for diseased or injured tendon.
Keywords: biomechanics; modulus; strain; stress; tendon.
Figures





Similar articles
-
Tendon material properties vary and are interdependent among turkey hindlimb muscles.J Exp Biol. 2012 Oct 15;215(Pt 20):3552-8. doi: 10.1242/jeb.072728. Epub 2012 Jul 5. J Exp Biol. 2012. PMID: 22771746 Free PMC article.
-
Effect of fiber orientation and strain rate on the nonlinear uniaxial tensile material properties of tendon.J Biomech Eng. 2003 Oct;125(5):726-31. doi: 10.1115/1.1614819. J Biomech Eng. 2003. PMID: 14618932
-
The biomechanical effects of limb lengthening and botulinum toxin type A on rabbit tendon.J Biomech. 2010 Dec 1;43(16):3177-82. doi: 10.1016/j.jbiomech.2010.07.032. Epub 2010 Aug 16. J Biomech. 2010. PMID: 20719314
-
"Soft, hard, or just right?" Applications and limitations of axial-strain sonoelastography and shear-wave elastography in the assessment of tendon injuries.Skeletal Radiol. 2014 Jan;43(1):1-12. doi: 10.1007/s00256-013-1695-3. Epub 2013 Aug 8. Skeletal Radiol. 2014. PMID: 23925561 Review.
-
Biomechanics and pathophysiology of overuse tendon injuries: ideas on insertional tendinopathy.Sports Med. 2004;34(14):1005-17. doi: 10.2165/00007256-200434140-00005. Sports Med. 2004. PMID: 15571430 Review.
Cited by
-
Caffeine intake does not appear to impair tendon-to-bone healing strength in a rat rotator cuff repair model.JSES Int. 2022 Jan 30;6(3):463-467. doi: 10.1016/j.jseint.2021.12.011. eCollection 2022 May. JSES Int. 2022. PMID: 35572424 Free PMC article.
-
Longitudinal Evidence for High-Level Patellar Tendon Strain as a Risk Factor for Tendinopathy in Adolescent Athletes.Sports Med Open. 2023 Sep 7;9(1):83. doi: 10.1186/s40798-023-00627-y. Sports Med Open. 2023. PMID: 37673828 Free PMC article.
-
Biodegradable polymer nanocomposites for ligament/tendon tissue engineering.J Nanobiotechnology. 2020 Jan 30;18(1):23. doi: 10.1186/s12951-019-0556-1. J Nanobiotechnology. 2020. PMID: 32000800 Free PMC article. Review.
-
Vitamin C-enriched gelatin supplementation before intermittent activity augments collagen synthesis.Am J Clin Nutr. 2017 Jan;105(1):136-143. doi: 10.3945/ajcn.116.138594. Epub 2016 Nov 16. Am J Clin Nutr. 2017. PMID: 27852613 Free PMC article. Clinical Trial.
-
Regional Patellar Tendon Strain in the Short- and Long-term After ACL Reconstruction Using Bone-Patellar Tendon-Bone Autograft.Am J Sports Med. 2025 Mar;53(3):632-639. doi: 10.1177/03635465241310152. Epub 2025 Jan 22. Am J Sports Med. 2025. PMID: 39838928
References
-
- Andreassen TT, Seyer-Hansen K, Bailey AJ. Thermal stability, mechanical properties and reducible cross-links of rat tail tendon in experimental diabetes. Biochim Biophys Acta 677: 313–317, 1981. - PubMed
-
- Awad HA, Boivin GP, Dressler MR, Smith FN, Young RG, Butler DL. Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res 21: 420–431, 2003 - PubMed
-
- Bailey AJ. Molecular mechanisms of ageing in connective tissues. Mech Ageing Dev 122: 735–755, 2001 - PubMed
-
- Batson EL, Paramour RJ, Smith TJ, Birch HL, Patterson-Kane JC, Goodship AE. Are the material properties and matrix composition of equine flexor and extensor tendons determined by their functions? Equine Vet J 35: 314–318, 2003 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

