Epithelial markers of colorectal carcinogenesis in ulcerative colitis and primary sclerosing cholangitis
- PMID: 23599650
- PMCID: PMC3627888
- DOI: 10.3748/wjg.v19.i14.2234
Epithelial markers of colorectal carcinogenesis in ulcerative colitis and primary sclerosing cholangitis
Abstract
Aim: To evaluate the expression of epithelial markers of colorectal carcinogenesis in patients with long-term ulcerative colitis (UC) and primary sclerosing cholangitis (PSC) before and after transplantation.
Methods: Eight patients with UC and PSC prior to liver transplantation (PSC-UC), 22 patients with UC after liver transplantation for PSC (OLT), 9 patients with active ulcerative colitis without PSC (UCA), 7 patients with UC in remission (UCR) and 10 controls (N) underwent colonoscopy with multiple biopsies. Specimens were analysed histologically and semi-quantitatively immunohistochemically for p53, Bcl-2 and cyclooxygenase-2 (COX-2) markers. Statistical analysis was performed by Kruskal-Wallis and Fisher's exact tests.
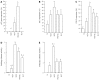
Results: PSC-UC had a statistically significantly higher expression of p53 in the nondysplastic mucosa as compared to OLT, UCA, UCR and N (P < 0.05). We also found a statistically significant positive correlation between the incidence of PSC and the expression of p53 (P < 0.001). UCA had a higher p53 expression as compared to UCR. OLT had a significantly lower expression of p53 as compared with PSC-UC (P < 0.001). Bcl-2 had a significant higher bcl-2 expression as compared with controls. No difference in COX-2 expression between PSC-UC, UCR and UCA was found. UCA had higher COX-2 expression as compared to UCR. We also found a statistically significant positive correlation between the expression of COX-2 and p53. Patients after liver transplantation for PSC had a statistically significantly lower expression of the p53 compared with PSC-UC (P < 0.001). PSC-UC had the same inflammatory endoscopic activity as OLT and UCR when evaluated with the Mayo score.
Conclusion: Our study shows that the nondysplatic mucosa of UC patients with PSC is characterised by a higher expression of the tumour suppressor gene p53, suggesting a higher susceptibility of cancer. This p53 overexpression correlates with the presence of PSC whilst it is not present in patients with UC after liver transplantation for PSC.
Keywords: Colorectal carcinoma; Cyclooxygenase-2; Imunohistochemistry; Liver transplantation; Primary sclerosing cholangitis; Ulcerative colitis; bcl2; p53.
Figures
References
-
- Weismüller TJ, Wedemeyer J, Kubicka S, Strassburg CP, Manns MP. The challenges in primary sclerosing cholangitis--aetiopathogenesis, autoimmunity, management and malignancy. J Hepatol. 2008;48 Suppl 1:S38–S57. - PubMed
-
- Torres J, Pineton de Chambrun G, Itzkowitz S, Sachar DB, Colombel JF. Review article: colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34:497–508. - PubMed
-
- Triantafillidis JK, Nasioulas G, Kosmidis PA. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009;29:2727–2737. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

