A Comparison of Stent Implant versus Medical Treatment for Severe Symptomatic Intracranial Stenosis: A Controlled Clinical Trial
- PMID: 23599702
- PMCID: PMC3567882
- DOI: 10.1159/000344004
A Comparison of Stent Implant versus Medical Treatment for Severe Symptomatic Intracranial Stenosis: A Controlled Clinical Trial
Abstract
Background: Atherosclerotic stenosis of the major intracranial arteries is the most common cause of ischemic stroke. There are limited treatments for severe intracranial stenosis, and stent placement versus medical treatment remains controversial. The aim of this study was to compare functional outcomes of these two modalities in patients with severe symptomatic intracranial stenosis.
Methods: At a single center, between 2008 and 2011, patients with angiographically demonstrated severe (70-90%) symptomatic intracranial atherosclerosis were divided into two groups: group A, which received only medical treatment, and group B, which underwent endovascular stent implant treatment. The severity and location of the stenosis was determined by digital subtraction angiography and the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) trial criteria in all patients. The exclusion criteria were: specific causes other than atherosclerosis, such as artery dissection, fibromuscular dysplasia, vasculitis, radiation and intracranial hemorrhage, focal neurological deficit that did not correlate to internal carotid artery or middle cerebral artery stenosis. All procedures were done under light anesthesia. Technical success was defined as the reduction of stenosis to <30% with complete enveloping of the lesion after the procedure. Early and late adverse events and functional outcomes were compared between the groups using the modified Rankin Scale (mRS).
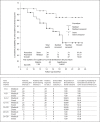
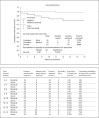
Results: Overall, 63 patients (29 in group A and 34 in group B) were evaluated and followed for a mean period of 15.22 months (range 6-25). The technical success rate was 97% in a total of 34 stents in 34 patients. There was no difference between the early (within 30 days) adverse event rates of the two groups. The median follow-up duration for the stent implant patients was 15 months (range 6-25), and for the medically treated cohort it was 14 months (range 8-25). The re-stenosis rate was 5.8% and the total number of late (>30 days) adverse events, including stroke, myocardial infarction and death, was 1 (2.9%) and 6 (20.7%) in the stent implant and medical groups, respectively (p = 0.042). The stent implant group had significantly better favorable functional outcomes according to the mRS than the medical group (93.9 vs. 63.0%). The cumulative secondary adverse event-free survival was significantly lower in the stent implant group.
Conclusion: Stent implants can be considered more durable and safe for patients with symptomatic severe stenosis of the internal carotid artery or middle cerebral artery, despite optimal medical therapy. Randomized, multicenter trials are required to confirm these results.
Keywords: Balloon-mounted coronary bare metal stents; Functional outcome; Intracranial stenosis; Ischemic stroke; Primary adverse events; Secondary adverse events; Self-expandable stents.
Figures
References
-
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. - PubMed
-
- Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, et al. Heart disease and stroke statistics – 2006 update. Circulation. 2006;113:e85–e151. - PubMed
-
- Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008;39:2396–2399. - PubMed
-
- Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. 2006;1:158–159. - PubMed
-
- Lau AY, Wong EH, Wong A, Mok VC, Leung TW, Wong KS. Significance of good collateral compensation in symptomatic intracranial atherosclerosis. Cerebrovasc Dis. 2012;33:517–524. - PubMed
LinkOut - more resources
Full Text Sources

