Comparison of the expression of 5 heat shock proteins in benign and malignant salivary gland tumor tissues
- PMID: 23599795
- PMCID: PMC3629267
- DOI: 10.3892/ol.2013.1166
Comparison of the expression of 5 heat shock proteins in benign and malignant salivary gland tumor tissues
Abstract
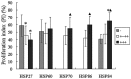
The objective of this study was to analyze the significance and potential value of heat shock proteins (HSPs) in salivary gland tumors. We found that expression of HSP60, HSP70, HSP86 and HSP84 were all upregulated in both salivary gland benign tumors and malignant tumors, and that the expression of HSP70, HSP86 and HSP84 was more greatly overexpressed in the malignant tumors (each P<0.01). For HSP27, expression was upregulated both in malignant and benign tumors, with less expression observed in malignant tumors (P<0.01). In malignant tumors, expression of HSP27 was negatively correlated with the age of the patients, size of the tumor tissue, occurrence of neural invasion and metastasis (each P<0.05). Additionally, in malignant tumors, HSP70 and HSP86 were both positively correlated with occurrence site, neural invasion and metastasis (each P<0.05), while HSP60 was only negatively correlated with the age of the patients (P<0.05). HSP86 was also positively correlated with malignant degree (P<0.01). In malignant tumors, the proliferation index (PI), which was marked by proliferating cell nuclear antigen (PCNA; PCNA-PI) was 49.95±14.569, which was significantly higher compared with that in benign tumors (P<0.001), which was in accordance with the upregulation of HSP70, HSP86 or HSP84; however, an adverse correlation was found between HSP27 expression and PCNA (each P<0.05). In conclusion, these results suggest that HSPs are involved in the occurrence and development of salivary gland tumors. HSP70, HSP86 and HSP84 retained the higher multiplication capability of the malignant tumor cells, however, HSP27 did not. Thus, the upregulation of HSP70, HSP86 and HSP84 and the downregulation of HSP27 may all be used as biomarkers of the occurrence and development of malignant salivary gland tumors. Moreover, the extremely high expression of HSP86 and HSP84 in benign tumors indicates the malignant transformation potential.
Keywords: heat shock proteins; proliferating cell nuclear antigen; salivary gland tumor; significance.
Figures



References
-
- Khalil AA, Kabapy NF, Deraz SF, Smith C. Heat shock proteins in oncology: diagnostic biomarkers or therapeutic targets? Biochim Biophys Acta. 2011;1816:89–104. - PubMed
-
- Rérole AL, Gobbo J, De TA, et al. Peptides and aptamers targeting HSP70: a novel approach for anticancer chemotherapy. Cancer Res. 2011;71:484–495. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
