Enzymology of H2S biogenesis, decay and signaling
- PMID: 23600844
- PMCID: PMC3910450
- DOI: 10.1089/ars.2013.5339
Enzymology of H2S biogenesis, decay and signaling
Abstract
Significance: Hydrogen sulfide (H2S), produced by the desulfuration of cysteine or homocysteine, functions as a signaling molecule in an array of physiological processes including regulation of vascular tone, the cellular stress response, apoptosis, and inflammation.
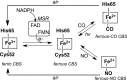
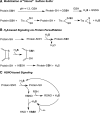
Recent advances: The low steady-state levels of H2S in mammalian cells have been recently shown to reflect a balance between its synthesis and its clearance. The subversion of enzymes in the cytoplasmic trans-sulfuration pathway for producing H2S from cysteine and/or homocysteine versus producing cysteine from homocysteine, presents an interesting regulatory problem.
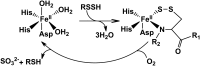
Critical issues: It is not known under what conditions the enzymes operate in the canonical trans-sulfuration pathway and how their specificity is switched to catalyze the alternative H2S-producing reactions. Similarly, it is not known if and whether the mitochondrial enzymes, which oxidize sulfide and persulfide (or sulfane sulfur), are regulated to increase or decrease H2S or sulfane-sulfur pools.
Future directions: In this review, we focus on the enzymology of H2S homeostasis and discuss H2S-based signaling via persulfidation and thionitrous acid.
Figures



References
-
- Akagi R. Purification and characterization of cysteine aminotransferase from rat liver cytosol. Acta Med Okayama 36: 187–197, 1982 - PubMed
-
- Bartholomew TC, Powell GM, Dodgson KS, and Curtis CG. Oxidation of sodium sulphide by rat liver, lungs and kidney. Biochem Pharmacol 29: 2431–2437, 1980 - PubMed
-
- Beatty PW. and Reed DJ. Involvement of the cystathionine pathway in the biosynthesis of glutathione by isolated rat hepatocytes. Arch Biochem Biophys 204: 80–87, 1980 - PubMed
-
- Birchmeier W. and Christen P. Syncatalytic enzyme modification: characteristic features and differentiation from affinity labeling. Methods Enzymol 46: 41–48, 1977 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

