Effects of maternal nutrient restriction, intrauterine growth restriction, and glucocorticoid exposure on phosphoenolpyruvate carboxykinase-1 expression in fetal baboon hepatocytes in vitro
- PMID: 23600855
- PMCID: PMC3979338
- DOI: 10.1111/jmp.12048
Effects of maternal nutrient restriction, intrauterine growth restriction, and glucocorticoid exposure on phosphoenolpyruvate carboxykinase-1 expression in fetal baboon hepatocytes in vitro
Abstract
Background: The objective of this study was to develop a cell culture system for fetal baboon hepatocytes and to test the hypotheses that (i) expression of the gluconeogenic enzyme phosphoenolpyruvate carboxykinase-1 (PEPCK-1) is upregulated in hepatocytes isolated from fetuses of nutrient-restricted mothers (MNR) compared with ad libitum-fed controls (CTR), and (ii) glucocorticoids stimulate PEPCK-1 expression.
Methods: Hepatocytes from 0.9G CTR and MNR fetuses were isolated and cultured. PEPCK-1 protein and mRNA levels in hepatocytes were determined by Western blot and quantitative PCR, respectively.
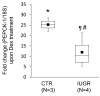
Results: Fetuses of MNR mothers were intrauterine growth restricted (IUGR). Feasibility of culturing 0.9G fetal baboon hepatocytes was demonstrated. PEPCK-1 protein levels were increased in hepatocytes isolated from IUGR fetuses, and PEPCK-1 mRNA expression was stimulated by glucocorticoids in fetal hepatocytes.
Conclusions: Cultured fetal baboon hepatocytes that retain their in vivo phenotype provide powerful in vitro tools to investigate mechanisms that regulate normal and programmed hepatic function.
Keywords: dexamethasone; diabetes non-human primate; liver.
© 2013 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures


References
-
- Abu Shehab M, Khosravi J, Han VKM, et al. Site-specific IGFBP-1 hyper-phosphorylation in fetal growth restriction: clinical and functional relence. J Proteome Res. 2010;9:1873–1881. - PubMed
-
- Armitage JA, Poston L, Taylor PD. Developmental origins of obesity and the metabolic syndrome: the role of maternal obesity. Front Horm Res. 2008;36:73–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

