Quantifying the mechanism of phosphate monoester hydrolysis in aqueous solution by evaluating the relevant ab initio QM/MM free-energy surfaces
- PMID: 23601038
- PMCID: PMC3797183
- DOI: 10.1021/jp4020146
Quantifying the mechanism of phosphate monoester hydrolysis in aqueous solution by evaluating the relevant ab initio QM/MM free-energy surfaces
Abstract
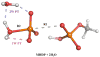
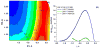
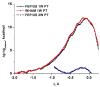
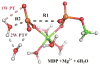
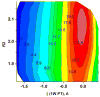
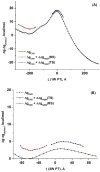
Understanding the nature of the free-energy surfaces for phosphate hydrolysis is a prerequisite for understanding the corresponding key chemical reactions in biology. Here, the challenge has been to move to careful ab initio QM/MM (QM(ai)/MM) free-energy calculations, where obtaining converging results is very demanding and computationally expensive. This work describes such calculations, focusing on the free-energy surface for the hydrolysis of phosphate monoesters, paying special attention to the comparison between the one water (1W) and two water (2W) paths for the proton-transfer (PT) step. This issue has been explored before by energy minimization with implicit solvent models and by nonsystematic QM/MM energy minimization, as well as by nonsystematic free-energy mapping. However, no study has provided the needed reliable 2D (3D) surfaces that are necessary for reaching concrete conclusions. Here we report a systematic evaluation of the 2D (3D) free-energy maps for several relevant systems, comparing the results of QM(ai)/MM and QM(ai)/implicit solvent surfaces, and provide an advanced description of the relevant energetics. It is found that the 1W path for the hydrolysis of the methyl diphosphate (MDP) trianion is 6-9 kcal/mol higher than that the 2W path. This difference becomes slightly larger in the presence of the Mg(2+) ion because this ion reduces the pKa of the conjugated acid form of the phosphate oxygen that accepts the proton. Interestingly, the BLYP approach (which has been used extensively in some studies) gives a much smaller difference between the 1W and 2W activation barriers. At any rate, it is worth pointing out that the 2W transition state for the PT is not much higher that the common plateau that serves as the starting point of both the 1W and 2W PT paths. Thus, the calculated catalytic effects of proteins based on the 2W PT mechanistic model are not expected to be different from the catalytic effects predicted using the 1W PT mechanistic model, which was calibrated on the observed barrier in solution and in which the TS charge distribution was similar to the that of the plateau (as was done in all of our previous EVB studies).
Figures


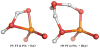









References
-
- Vetter IR, Wittinghofer A. Nucleoside Triphosphate-Binding Proteins: Different Scaffolds to Achieve Phosphoryl Transfer. Quarterly Reviews of Biophysics. 1999;32:1–56. - PubMed
-
- Cleland WW, Hengge AC. Enzymatic Mechanisms of Phosphate and Sulfate Transfer. Chem Rev. 2006;106:3252–3278. - PubMed
-
- Benkovic SJ, Schray KJ. Academic Press; New York: 1973. pp. 201–238.
-
- Cox JR, Jr, Ramsay OB. Mechanisms of Nucleophilic Substitution in Phosphate Esters. Chem Rev. 1964;64:317–352.
-
- Kirby JA, Warren SG. The Organic Chemistry of Phosphorus. Elsevier; Amsterdam: 1967.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

