The greasy response to virus infections
- PMID: 23601099
- PMCID: PMC3654690
- DOI: 10.1016/j.chom.2013.04.002
The greasy response to virus infections
Abstract
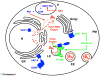
Virus replication requires lipid metabolism, but how lipids mediate virus infection remains obscure. In this issue, Amini-Bavil-Olyaee et al. (2013) reveal that IFITM proteins disturb cholesterol homeostasis to block virus entry. Previously, in Cell, Morita and colleagues (2013) showed the antiviral potency of the lipid mediator protectin D1.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
Comment on
-
The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza.Cell. 2013 Mar 28;153(1):112-25. doi: 10.1016/j.cell.2013.02.027. Epub 2013 Mar 7. Cell. 2013. PMID: 23477864
-
The antiviral effector IFITM3 disrupts intracellular cholesterol homeostasis to block viral entry.Cell Host Microbe. 2013 Apr 17;13(4):452-64. doi: 10.1016/j.chom.2013.03.006. Cell Host Microbe. 2013. PMID: 23601107 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

