Virus infections in the nervous system
- PMID: 23601101
- PMCID: PMC3647473
- DOI: 10.1016/j.chom.2013.03.010
Virus infections in the nervous system
Abstract
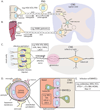
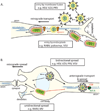
Virus infections usually begin in peripheral tissues and can invade the mammalian nervous system (NS), spreading into the peripheral (PNS) and more rarely the central (CNS) nervous systems. The CNS is protected from most virus infections by effective immune responses and multilayer barriers. However, some viruses enter the NS with high efficiency via the bloodstream or by directly infecting nerves that innervate peripheral tissues, resulting in debilitating direct and immune-mediated pathology. Most viruses in the NS are opportunistic or accidental pathogens, but a few, most notably the alpha herpesviruses and rabies virus, have evolved to enter the NS efficiently and exploit neuronal cell biology. Remarkably, the alpha herpesviruses can establish quiescent infections in the PNS, with rare but often fatal CNS pathology. Here we review how viruses gain access to and spread in the well-protected CNS, with particular emphasis on alpha herpesviruses, which establish and maintain persistent NS infections.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


References
-
- Tirabassi RS, et al. Molecular mechanisms of neurotropic herpesvirus invasion and spread in the CNS. Neurosci Biobehav Rev. 1998;22(6):709–720. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

