Eight weeks of supplementation with a multi-ingredient weight loss product enhances body composition, reduces hip and waist girth, and increases energy levels in overweight men and women
- PMID: 23601452
- PMCID: PMC3639826
- DOI: 10.1186/1550-2783-10-22
Eight weeks of supplementation with a multi-ingredient weight loss product enhances body composition, reduces hip and waist girth, and increases energy levels in overweight men and women
Abstract
Background: Numerous natural products are marketed and sold claiming to decrease body weight and fat, but few undergo finished product-specific research demonstrating their safety and efficacy.
Objective: To determine the safety and efficacy of a multi-ingredient supplement containing primarily raspberry ketone, caffeine, capsaicin, garlic, ginger and Citrus aurantium (Prograde Metabolism™ [METABO]) as an adjunct to an eight-week weight loss program.
Methods: Using a randomized, placebo-controlled, double-blind design, 70 obese but otherwise healthy subjects were randomly assigned to METABO or a placebo and underwent 8 weeks of daily supplementation, a calorie restricted diet, and exercise training. Subjects were tested for changes in body composition, serum adipocytokines (adiponectin, resistin, leptin, TNF-α, IL-6) and markers of health including heart rate and blood pressure.
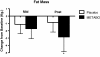
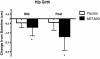
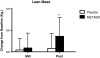
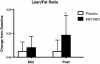
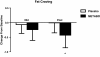
Results: Of the 45 subjects who completed the study, significant differences were observed in: body weight (METABO -2.0% vs. placebo -0.5%, P < 0.01), fat mass (METABO -7.8 vs. placebo -2.8%, P < 0.001), lean mass (METABO +3.4% vs. placebo +0.8%, P < 0.03), waist girth (METABO -2.0% vs. placebo -0.2%, P < 0.0007), hip girth (METABO -1.7% vs. placebo -0.4%, P < 0.003), and energy levels per anchored visual analogue scale (VAS) (METABO +29.3% vs. placebo +5.1%, P < 0.04). During the first 4 weeks, effects/trends for maintaining elevated serum leptin (P < 0.03) and decreased serum resistin (P < 0.08) in the METABO group vs. placebo were also observed. No changes in systemic hemodynamics, clinical blood chemistries, adverse events, or dietary intake were noted between groups.
Conclusions: METABO administration is a safe and effective adjunct to an eight-week diet and exercise weight loss program by augmenting improvements in body composition, waist and hip girth. Adherence to the eight-week weight loss program also led to beneficial changes in body fat in placebo. Ongoing studies to confirm these results and clarify the mechanisms (i.e., biochemical and neuroendocrine mediators) by which METABO exerts the observed salutary effects are being conducted.
Figures



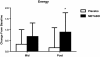
Similar articles
-
The Combined Effects of Exercise, Diet, and a Multi-Ingredient Dietary Supplement on Body Composition and Adipokine Changes in Overweight Adults.J Am Coll Nutr. 2018 Feb;37(2):111-120. doi: 10.1080/07315724.2017.1368039. Epub 2017 Nov 7. J Am Coll Nutr. 2018. PMID: 29111889 Clinical Trial.
-
Effects of a stimulant-free dietary supplement on body weight and fat loss in obese adults: a six-week exploratory study.Curr Ther Res Clin Exp. 2003 Apr;64(4):248-62. doi: 10.1016/S0011-393X(03)00058-4. Curr Ther Res Clin Exp. 2003. PMID: 24944372 Free PMC article.
-
Sibutramine is effective for weight loss and diabetic control in obesity with type 2 diabetes: a randomised, double-blind, placebo-controlled study.Diabetes Obes Metab. 2000 Apr;2(2):105-12. doi: 10.1046/j.1463-1326.2000.00071.x. Diabetes Obes Metab. 2000. PMID: 11220522 Clinical Trial.
-
Effect of lean system 7 on metabolic rate and body composition.Nutrition. 2005 Feb;21(2):179-85. doi: 10.1016/j.nut.2004.05.025. Nutrition. 2005. PMID: 15723746 Clinical Trial.
-
Citrus aurantium as a thermogenic, weight-reduction replacement for ephedra: an overview.J Med. 2002;33(1-4):247-64. J Med. 2002. PMID: 12939122 Review.
Cited by
-
Pharmacological Exploration of Phenolic Compound: Raspberry Ketone-Update 2020.Plants (Basel). 2021 Jun 29;10(7):1323. doi: 10.3390/plants10071323. Plants (Basel). 2021. PMID: 34209554 Free PMC article. Review.
-
Sex-Specific Effects on Total Body Fat Gain with 4-Week Daily Dosing of Raspberry Ketone [4-(4-Hydroxyphenyl)-2-butanone] and Ketogenic Diet in Mice.Nutrients. 2023 Mar 28;15(7):1630. doi: 10.3390/nu15071630. Nutrients. 2023. PMID: 37049471 Free PMC article.
-
Lipid-Lowering Efficacy of the Capsaicin in Patients With Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.Front Nutr. 2022 Mar 1;9:812294. doi: 10.3389/fnut.2022.812294. eCollection 2022. Front Nutr. 2022. PMID: 35299764 Free PMC article.
-
Capsaicinoids: a spicy solution to the management of obesity?Int J Obes (Lond). 2016 Aug;40(8):1198-204. doi: 10.1038/ijo.2015.253. Epub 2015 Dec 21. Int J Obes (Lond). 2016. PMID: 26686003 Review.
-
Comparison of Dual-Energy X-ray Absorptiometry (DXA) Versus a Multi-Frequency Bioelectrical Impedance (InBody 770) Device for Body Composition Assessment after a 4-Week Hypoenergetic Diet.J Funct Morphol Kinesiol. 2019 Apr 25;4(2):23. doi: 10.3390/jfmk4020023. J Funct Morphol Kinesiol. 2019. PMID: 33467338 Free PMC article.
References
-
- Dixon JB. The effect of obesity on health outcomes. Mol Cell Endocrinol. 2009;316:104–108. - PubMed
-
- Adult Obesity Facts, Centers for Disease Control and Prevention. http://www.cdc.gov/obesity/data/adult.html.
-
- Metabolic Syndrome, MedinePlus. http://www.nlm.nih.gov/medlineplus/metabolicsyndrome.html.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
