Bio-fabrication of gold nanoparticles using aqueous extract of red tomato and its use as a colorimetric sensor
- PMID: 23601560
- PMCID: PMC3663728
- DOI: 10.1186/1556-276X-8-181
Bio-fabrication of gold nanoparticles using aqueous extract of red tomato and its use as a colorimetric sensor
Abstract
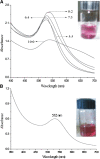
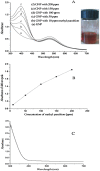
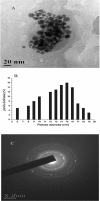
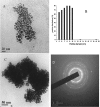
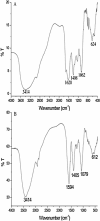
In this work, we report a green method for the synthesis of gold nanoparticles (GNP) using the aqueous extract of red tomato (Lycopersicon esculentum). We believe that citric acid and ascorbic acid present in tomato juice are responsible for the reduction of gold ions. This biosynthesized GNP in the presence of sodium dodecyl sulfate has been used as a colorimetric sensor to detect and estimate the pesticide, methyl parathion. The GNP in the presence of methyl parathion shows a new peak at 400 nm due to the formation of 4-nitrophenolate ion by catalytic hydrolysis of methyl parathion in alkaline medium. A calibration curve between the absorption coefficients of the 400-nm peak versus the concentration of the pesticide allows the quantitative estimation of the 4-nitrophenolate ion, thereby enabling indirect estimation of methyl parathion present in the system.
Figures
Similar articles
-
Kinetic analysis of the hydrolysis of methyl parathion using citrate-stabilized 10 nm gold nanoparticles.Chemosphere. 2016 Feb;144:1916-9. doi: 10.1016/j.chemosphere.2015.10.036. Epub 2015 Nov 11. Chemosphere. 2016. PMID: 26547026
-
A Simple and Green Route for Room-Temperature Synthesis of Gold Nanoparticles and Selective Colorimetric Detection of Cysteine.J Food Sci. 2015 Sep;80(9):N2071-8. doi: 10.1111/1750-3841.12974. Epub 2015 Aug 3. J Food Sci. 2015. PMID: 26239641
-
A novel colorimetric sensor for measuring hydroperoxide content and peroxyl radical scavenging activity using starch-stabilized gold nanoparticles.Talanta. 2019 May 1;196:32-38. doi: 10.1016/j.talanta.2018.12.022. Epub 2018 Dec 11. Talanta. 2019. PMID: 30683370
-
Highly selective, sensitive and simpler colorimetric sensor for Fe2+ detection based on biosynthesized gold nanoparticles.Spectrochim Acta A Mol Biomol Spectrosc. 2021 Jun 5;254:119645. doi: 10.1016/j.saa.2021.119645. Epub 2021 Mar 9. Spectrochim Acta A Mol Biomol Spectrosc. 2021. PMID: 33744706
-
Colorimetric determination of F-, Br- and I- ions by Ehrlich's bio-reagent oxidation over enzyme mimic like gold nanoparticles: Peroxidase-like activity and multivariate optimization.Spectrochim Acta A Mol Biomol Spectrosc. 2020 Feb 5;226:117606. doi: 10.1016/j.saa.2019.117606. Epub 2019 Oct 9. Spectrochim Acta A Mol Biomol Spectrosc. 2020. PMID: 31614272
Cited by
-
Mechanism and Antibacterial Activity of Gold Nanoparticles (AuNPs) Functionalized with Natural Compounds from Plants.Pharmaceutics. 2022 Nov 25;14(12):2599. doi: 10.3390/pharmaceutics14122599. Pharmaceutics. 2022. PMID: 36559093 Free PMC article. Review.
-
Highly sensitive label-free bio-interfacial colorimetric sensor based on silk fibroin-gold nanocomposite for facile detection of chlorpyrifos pesticide.Sci Rep. 2020 Mar 6;10(1):4198. doi: 10.1038/s41598-020-61130-y. Sci Rep. 2020. PMID: 32144298 Free PMC article.
-
Cow dung extract mediated green synthesis of zinc oxide nanoparticles for agricultural applications.Sci Rep. 2022 Nov 27;12(1):20371. doi: 10.1038/s41598-022-22099-y. Sci Rep. 2022. PMID: 36437253 Free PMC article.
-
Synthesis of silver and gold nanoparticles using Jasminum nervosum leaf extract and its larvicidal activity against filarial and arboviral vector Culex quinquefasciatus Say (Diptera: Culicidae).Environ Sci Pollut Res Int. 2015 Nov;22(22):17753-68. doi: 10.1007/s11356-015-5001-x. Epub 2015 Jul 9. Environ Sci Pollut Res Int. 2015. PMID: 26154045
References
-
- Pal T, Sau TK, Jana NR. Reversible formation and dissolution of silver nanoparticles in aqueous surfactant media. Langmuir. 1997;8:1481–1485. doi: 10.1021/la960834o. - DOI
-
- Goia DV, Matijevic E. Formation mechanisms of uniform colloid particles. New J Chem. 1998;8:1203–1215. doi: 10.1039/a709236i. - DOI
-
- Munro CH, Smith WE, Garner M, Clarkson J, White PC. Characterization of the surface of a citrate-reduced colloid optimized for use as a substrate for surface-enhanced resonance Raman scatterings. Langmuir. 2002;8:3712–3720.
-
- Esumi K, Tano T, Torigoe K, Meguro K. Preparation and characterization of bimetallic palladium-copper colloids by thermal decomposition of their acetate compounds in organic solvents. Chem mater. 1990;8:564–587. doi: 10.1021/cm00011a019. - DOI
-
- Rodriguez-Sanchez ML, Blanco MC, Lopez-Quintela MA. Electrochemical synthesis of silver nanoparticles. J Phys Chem B. 2000;8:9683–9688. doi: 10.1021/jp001761r. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

