Tau phosphorylation affects its axonal transport and degradation
- PMID: 23601672
- PMCID: PMC3684773
- DOI: 10.1016/j.neurobiolaging.2013.03.015
Tau phosphorylation affects its axonal transport and degradation
Abstract
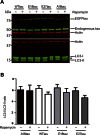
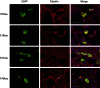
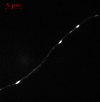
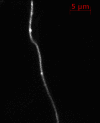
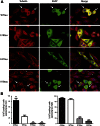
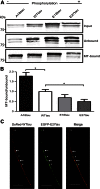
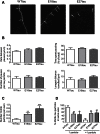
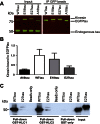
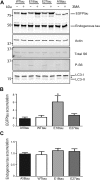
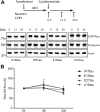
Phosphorylated forms of microtubule-associated protein tau accumulate in neurofibrillary tangles in Alzheimer's disease. To investigate the effects of specific phosphorylated tau residues on its function, wild type or phosphomutant tau was expressed in cells. Elevated tau phosphorylation decreased its microtubule binding and bundling, and increased the number of motile tau particles, without affecting axonal transport kinetics. In contrast, reducing tau phosphorylation enhanced the amount of tau bound to microtubules and inhibited axonal transport of tau. To determine whether differential tau clearance is responsible for the increase in phosphomimic tau, we inhibited autophagy in neurons which resulted in a 3-fold accumulation of phosphomimic tau compared with wild type tau, and endogenous tau was unaffected. In autophagy-deficient mouse embryonic fibroblasts, but not in neurons, proteasomal degradation of phosphomutant tau was also reduced compared with wild type tau. Therefore, autophagic and proteasomal pathways are involved in tau degradation, with autophagy appearing to be the primary route for clearing phosphorylated tau in neurons. Defective autophagy might contribute to the accumulaton of tau in neurodegenerative diseases.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Rho-kinase ROCK inhibitors reduce oligomeric tau protein.Neurobiol Aging. 2020 May;89:41-54. doi: 10.1016/j.neurobiolaging.2019.12.009. Epub 2019 Dec 16. Neurobiol Aging. 2020. PMID: 31982202 Free PMC article.
-
Autophagic Pathways to Clear the Tau Aggregates in Alzheimer's Disease.Cell Mol Neurobiol. 2021 Aug;41(6):1175-1181. doi: 10.1007/s10571-020-00897-0. Epub 2020 Jun 11. Cell Mol Neurobiol. 2021. PMID: 32529542 Free PMC article. Review.
-
Disrupted ubiquitin proteasome system underlying tau accumulation in Alzheimer's disease.Neurobiol Aging. 2021 Mar;99:79-85. doi: 10.1016/j.neurobiolaging.2020.11.015. Epub 2020 Dec 8. Neurobiol Aging. 2021. PMID: 33422896 Review.
-
Neurofibrillary tangles and tau phosphorylation.Biochem Soc Symp. 2001;(67):81-8. doi: 10.1042/bss0670081. Biochem Soc Symp. 2001. PMID: 11447842
-
Tyrosine 394 is phosphorylated in Alzheimer's paired helical filament tau and in fetal tau with c-Abl as the candidate tyrosine kinase.J Neurosci. 2005 Jul 13;25(28):6584-93. doi: 10.1523/JNEUROSCI.1487-05.2005. J Neurosci. 2005. PMID: 16014719 Free PMC article.
Cited by
-
Truncated tau interferes with the autophagy and endolysosomal pathway and results in lipid accumulation.Cell Mol Life Sci. 2024 Jul 15;81(1):304. doi: 10.1007/s00018-024-05337-6. Cell Mol Life Sci. 2024. PMID: 39009859 Free PMC article.
-
Triggering Innate Immune Receptors as New Therapies in Alzheimer's Disease and Multiple Sclerosis.Cells. 2021 Aug 22;10(8):2164. doi: 10.3390/cells10082164. Cells. 2021. PMID: 34440933 Free PMC article. Review.
-
In Vitro Assessment of the Neuroprotective Effects of Pomegranate (Punica granatum L.) Polyphenols Against Tau Phosphorylation, Neuroinflammation, and Oxidative Stress.Nutrients. 2024 Oct 28;16(21):3667. doi: 10.3390/nu16213667. Nutrients. 2024. PMID: 39519499 Free PMC article.
-
Metabolic disorder in Alzheimer's disease.Metab Brain Dis. 2021 Jun;36(5):781-813. doi: 10.1007/s11011-021-00673-z. Epub 2021 Feb 27. Metab Brain Dis. 2021. PMID: 33638805 Review.
-
The Ambiguous Relationship of Oxidative Stress, Tau Hyperphosphorylation, and Autophagy Dysfunction in Alzheimer's Disease.Oxid Med Cell Longev. 2015;2015:352723. doi: 10.1155/2015/352723. Epub 2015 Jun 15. Oxid Med Cell Longev. 2015. PMID: 26171115 Free PMC article. Review.
References
-
- Aplin A., Jasionowski T., Tuttle D.L., Lenk S.E., Dunn W.A., Jr. Cytoskeletal elements are required for the formation and maturation of autophagic vacuoles. J. Cell Physiol. 1992;152:458–466. - PubMed
-
- Ballatore C., Lee V.M., Trojanowski J.Q. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat. Rev. Neurosci. 2007;8:663–672. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

